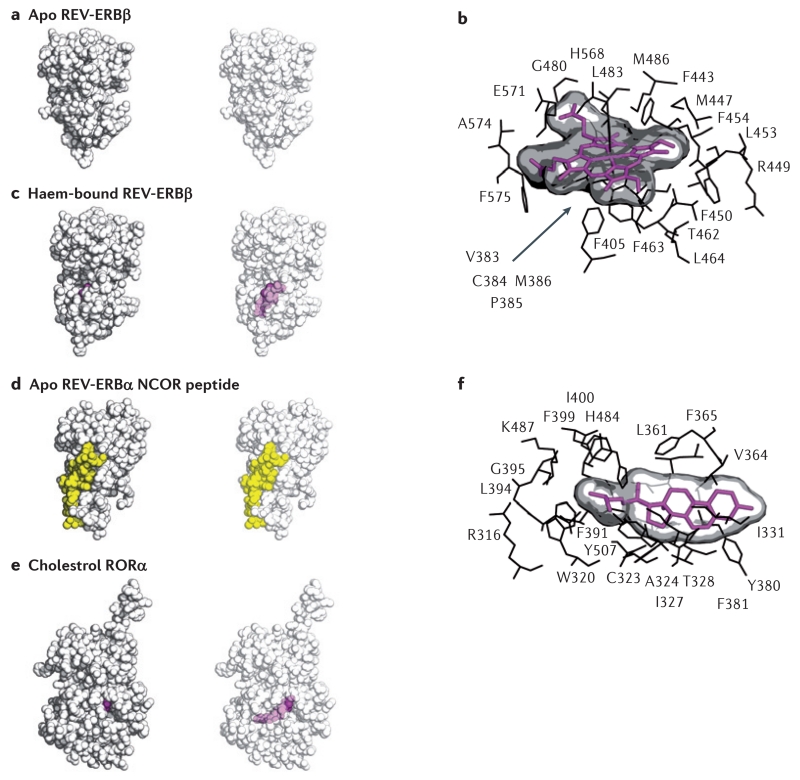
Figure 4. Structures of REV-ERB and ROR demonstrate their capacity to bind to natural ligands.
The apo structure of REV-ERBβ (part a) indicated that the putative ligand-binding pocket was filled with large hydrophobic residues and thus devoid of the space that would be necessary for a ligand to bind. However, the haem-bound REV-ERBβ structure (parts b and c) shows that its ligand-binding pocket can profoundly change its shape to accommodate haem, a large porphyrin natural ligand. Intriguingly, although studies indicate that the binding of haem to REV-ERB increases its interaction with the nuclear receptor co-repressor (NCOR)11,61, the structure of apo REV-ERB bound to an NCOR peptide (part d) indicates that the binding of haem may not be an absolute requirement for mediating the REV-ERB–NCOR interaction. The co-crystal structure of the ligand-binding domain of retinoic acid receptor-related orphan receptor-α (RORα) bound to cholesterol (parts e and f) sets the stage for other studies indicating that various cholesterol derivatives, such as 7-oxygenated sterols, may act as physiological ligands to influence ROR activity. Structures are shown as space-filling models (parts a, c, d and e), with and without transparency to allow visualization of ligands bound to the internal ligand-binding pocket (haem-bound REV-ERBβ and cholesterol-bound RORα). A snapshot of the residues mediating the interaction of haem with REV-ERB (part b) illustrates that the repositioned hydrophobic residues that were originally thought to block the ligand-binding pocket in fact cooperate in binding to the large hydrophobic porphyrin haem scaffold. Protein Data Bank (PDB) codes: apo REV-ERB, 2V0V; haem-bound REV-ERB, 3CQV; apo REV-ERB with an NCOR fragment (co-repressor nuclear receptor (CoRNR) box motif peptide, 3N00; cholesterol-bound ROR, 1N83.