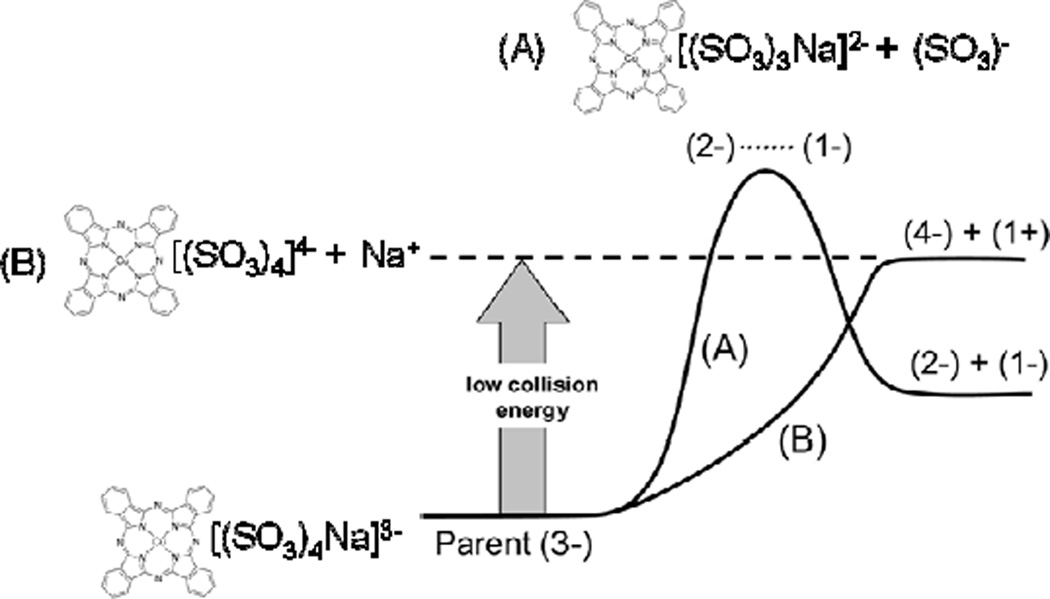
Figure 2.
Illustration of the homolytic and heterolytic dissociation channels for triply-charged [Cu phthalocyanine (SO3)4Na]3−. Dissociation along thermodynamically favored channel A (homolytic) is accessible only at high collision energies, due to its electrostatic kinetic barrier. Low collision energy dissociations access the more endothermic, barrierless channel B. (Modified from J. Am. Soc. Mass Spectrom., Vol. 19, S. Hashemi, M. J. Y. Jarvis, and D. K. Bohme, 375–379 (2008), with permission from American Society for Mass Spectrometry.)