Figure 2.
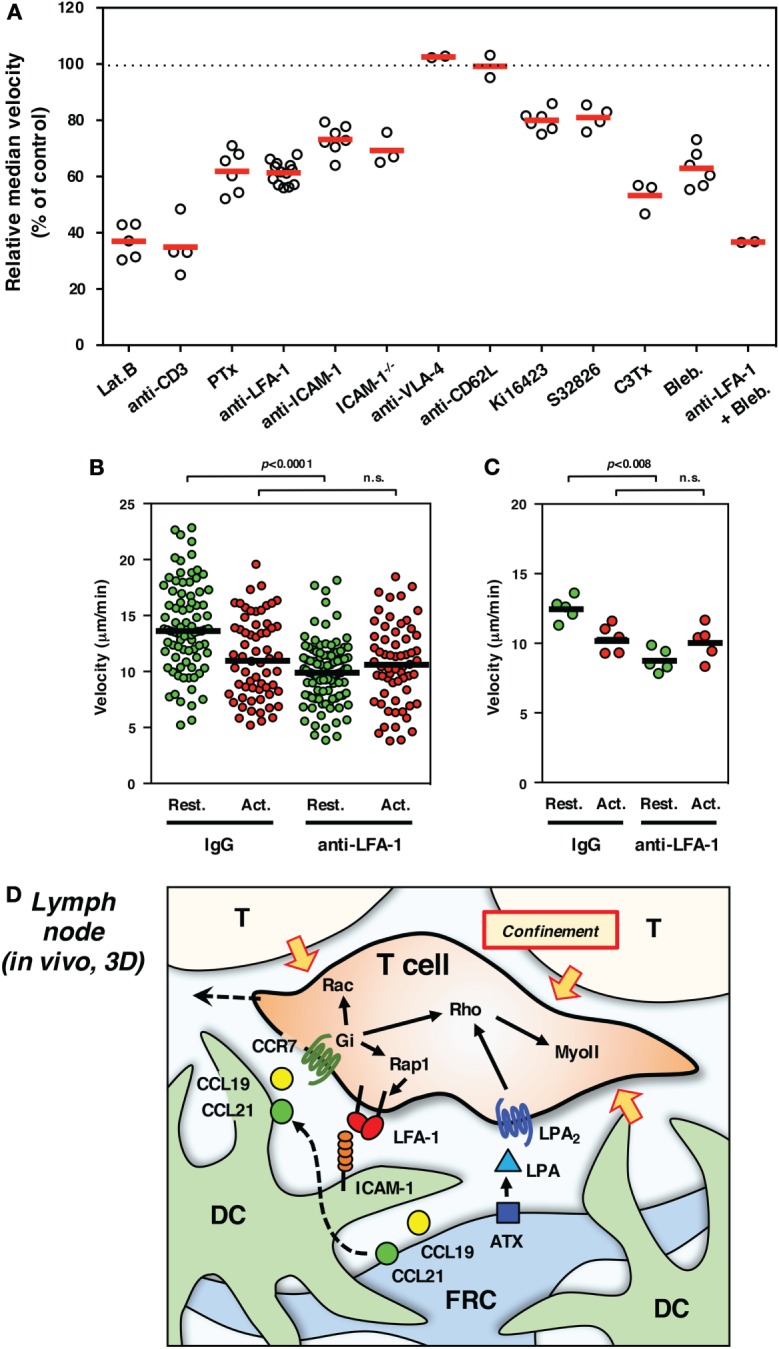
Interstitial T cell migration in LN. (A) Various treatments affect T cell migration in LN slices [partially adapted and modified from Ref. (27, 54)]. The circles represent the migration velocity (%) compared to the control in each experiment, and the horizontal red bars represent the mean (n ≧ 2). Lat.B, latrunclin B (actin inhibitor); Ki16425, LPAR inhibitor; S32826, ATX inhibitor; C3Tx, C3 toxin (Rho inhibitor); Bleb., blebbistatin (nmMyoII inhibitor). (B,C) Migration velocities of resting (Rest., green) and activated (Act., red) T cells in LN slices in the presence of control IgG or anti-LFA-1 antibody. The plot in (B) shows the mean velocity of individual cells (circles) and the median (horizontal bars), while the plot in (C) shows the median velocity for individual experiments (circles) and the mean of five experiments (n = 5, horizontal bars). For activation, total T cells isolated from LNs were stimulated with immobilized anti-CD3 and soluble anti-CD28 antibodies for 3 days. Freshly isolated resting T cells and activated T cells were labeled with different fluorescent dyes (CFSE and CMTMR), mixed at equal numbers, and applied to LN slices for the examination by two-photon laser scanning microscopy. The trajectory data sets of resting and activated T cells in each treatment were obtained from the same image field. Note that resting T cells but not activated T cells show the reduction of velocity in response to LFA-1 blockade, suggesting a distinct difference for LFA-1-dependent “speed-up” in resting but not activated T cells. Statistical analysis: Mann–Whitney U test. n.s., not significant. (D) Schematic representation of microenvironmental cues for high-speed interstitial T cell migration in the LN paracortex. FRCs produce chemokines (CCL19 and CCL21) and ATX (LPA), while DCs produce CCL19 but bind FRC-derived CCL21 on surface glycans. The CCR7 ligands input migratory signals in T cell, which induce actin reorganization, front–rear asymmetry, actomyosin contraction, and LFA-1-dependent dynamic adhesion to ICAM-1 on DC through the function of small GTPases. LPA plays additive or compensatory role to stimulate actomyosin-mediated motility and cellular deformation to adopt the complicated geometry and confinement of tissue microenvironment.
