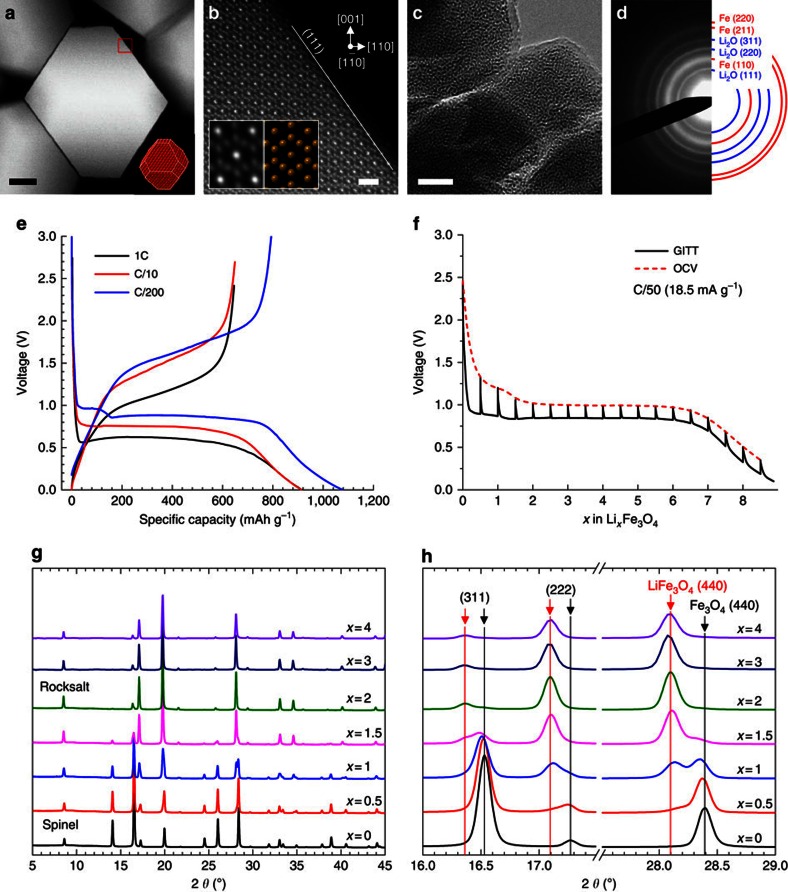
Figure 1. Electrode materials and electrochemical performance.
(a) STEM image of the pristine Fe3O4 single crystals. Inset showing the crystals in the truncated octahedral shape. (b) High-resolution HAADF-STEM image showing spinel structure along [1–10] zone axis. Inset showing enlarged STEM image compared with the atom model. (c) TEM image and (d) SAED pattern of the fully lithiated electrode, confirming the nanocomposite of Fe nanocrystals in amorphous Li2O matrix. Scale bars, (a,c) 20 nm; (b) 1 nm. (e) First-cycle charge/discharge profiles of coin-cell batteries at rates of 1C, C/10 and C/200 (that is, 926 mA g−1, 92.6 mA g−1 and 4.63 mA g−1). (f) GITT and OCV profiles in the first discharge cycle measured at C/50 (18.5 mA g−1). GITT measurements were performed by applying an intermittent current of C/50 for 3.125 h followed by a 24 h relaxation period. (g) SXRD patterns of LixFe3O4 at various SOCs (x=0, 0.5, 1, 1.5, 2, 3, 4). (h) Enlarged SXRD patterns showing detailed peak shifts of (311), (222) and (440) reflections. Fe3O4 and LiFe3O4 phases are marked by black and red arrows. X-ray wavelength is 0.72768 Å.