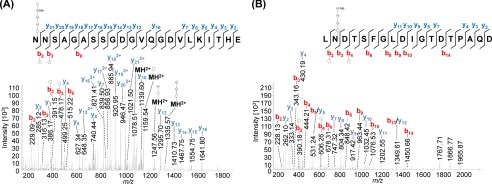
FIGURE 4.
Mass spectrometric identification of N-glycosylated peptides from PilA1. Peptides of GluC-digested proteins from CsCl density gradients obtained from WT cells were analyzed by MS. HCD fragmentation of intact glycopeptides allowed the identification of the peptide sequence by analyzing the resulting fragment ions, although the mass difference between the peptide backbone and the precursor ion led to the identification of the glycan composition. MS2 spectra corresponding to the fragmentation of N@NSAGASSGDGVQGDVLKITHE (A, precursor m/z 1008.74, charge 3) and LN@DTSFGLDIGTDTPAQD (B, precursor m/z 1292.51, charge 2) from PilA1 show the modification with a penta- and tetrasaccharide, respectively, at the marked glycosylation sites. An overview about the observed N-glycans with varying lengths is shown in Table 4. The observed b-ions (red) and y-ions (blue) are indicated in the spectra as well as the fragmentation scheme, including glycan fragments composed of hexose (○), hexuronic acid (♢), and methylated hexuronic acid (♢Me) that were attached to the peptide or fragment ions (n = 2).