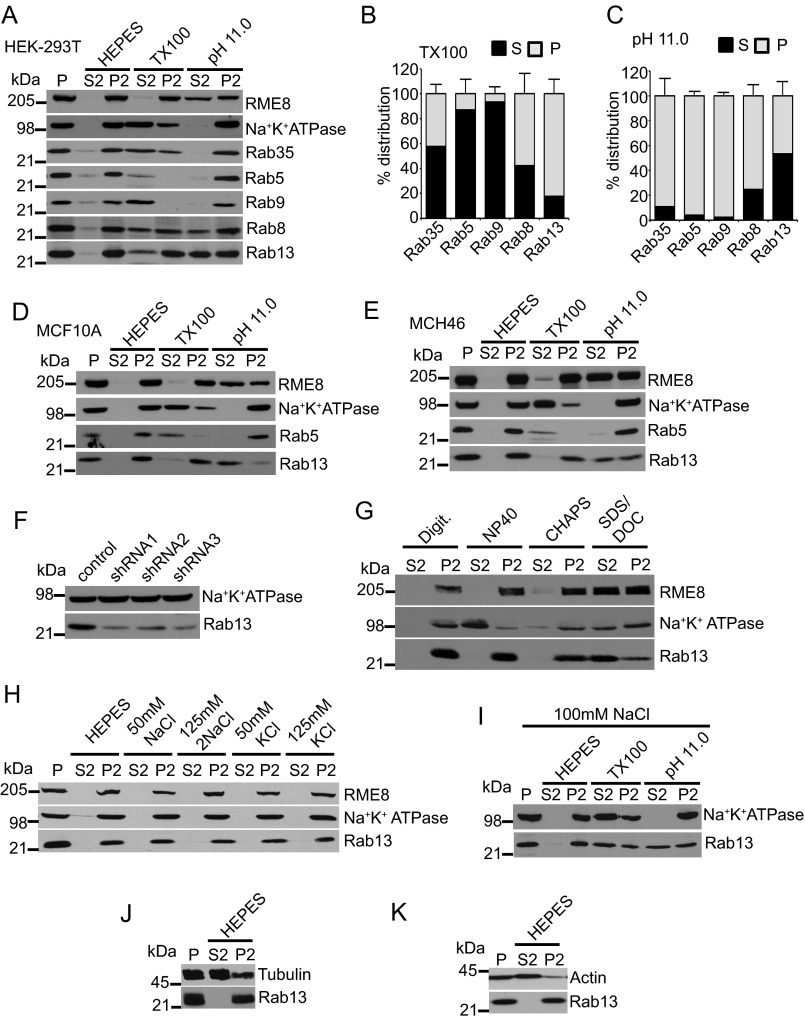
FIGURE 5.
Rab13 associates with membranes via protein-protein interactions in cells. A, HEK-293T cell homogenates in HEPES buffer were spun for 30 min at 200,000 × g, and equal protein aliquots of the resulting pellet (P) were resuspended in ice-cold HEPES buffer with or without 1% Triton X-100 (TX100) or NaCO3 at pH 11. 0. After 15 min of incubation, samples were spun for 30 min at 200,000 × g, and the resulting supernatant (S2) and pellet (P2) fractions were analyzed by Western blotting using the indicated antibodies. B and C, quantification of the distribution of Rab proteins resuspended in Triton X-100 (B) and at pH 11.0 (C). Data are mean ± S.D. (n = 7 for Rab13, n = 5 for Rab5, n = 4 for Rab8 and Rab35, and n = 3 for Rab9). D--E, lysates of MCF10A cells (D) and MCH46 cells (E) were processed and analyzed as in A. F, HEK-293T cells were stably transduced with a lentivirus driving the expression of control shRNA or three different shRNAs targeting Rab13, and the indicated proteins were detected by blotting. G, HEK-293T cell homogenates in HEPES buffer were spun for 30 min at 200,000 × g, and equal protein aliquots of the resulting pellet were resuspended in ice-cold HEPES buffer with digitonin (Digit.), Nonidet P-40, CHAPS, or SDS/deoxycholate (DOC). After 15 min of incubation, samples were spun for 30 min at 200,000 × g, and the resulting supernatant and pellet fractions were analyzed by Western blotting using the indicated antibodies. H, HEK-293T cell homogenates in HEPES buffer were spun for 30 min at 200,000 × g, and equal protein aliquots of the resulting pellet were resuspended in ice-cold HEPES buffer with or without NaCl or KCl at the indicated concentrations. After 15 min of incubation, the samples were spun for 30 min at 200,000 × g, and the resulting supernatant and pellets were analyzed by Western blotting using the indicated antibodies. I, HEK-293T cell homogenates in HEPES buffer were spun for 30 min at 200,000 × g, and equal protein aliquots of the resulting pellet were resuspended in ice-cold HEPES buffer containing 100 mm NaCl with or without 1% Triton X-100 or NaCO3 at pH 11.0. After 15 min of incubation, samples were spun for 30 min at 200,000 × g, and the resulting supernatant and pellet fractions were analyzed by Western blotting using the indicated antibodies. J and K, HEK-293T cell homogenates in HEPES buffer were spun for 30 min at 200,000 × g, and equal protein aliquots of the resulting pellet were resuspended in ice-cold HEPES buffer. After 15 min of incubation, samples were spun for 30 min at 200,000 × g, and the resulting supernatant and pellets were analyzed by Western blotting using the indicated antibodies.