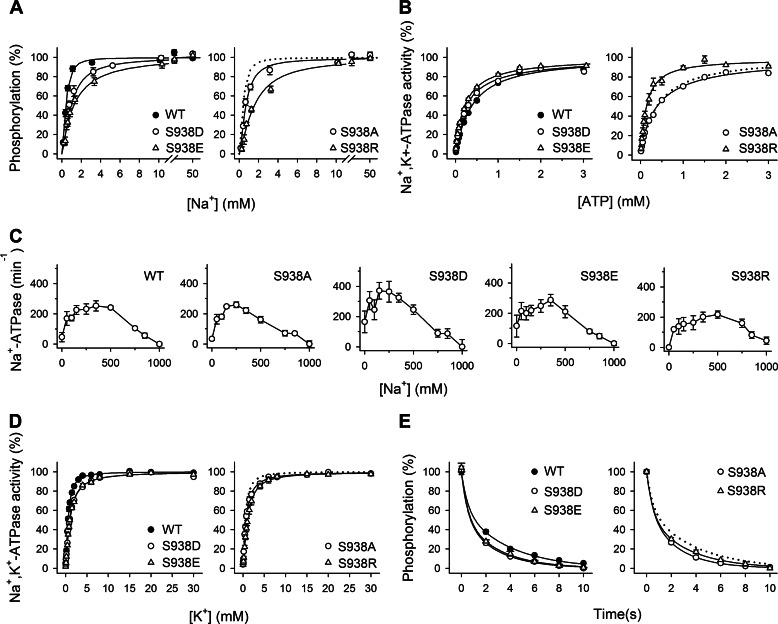
FIGURE 2.
The phosphomimetic S938D/E mutations interfere with the binding of Na+ at the intracellularly facing E1 sites. A, Na+ dependence of phosphorylation from [γ-32P]ATP. Each line shows the best fit of the Hill equation, and the extracted K0.5 values are listed in Table 1. B, ATP dependence of Na+,K+-ATPase activity. Each line shows the best fit of the Hill equation, and the extracted K0.5 values are listed in Table 1. C, Na+ dependence of Na+-ATPase activity. Inhibition of Na+-ATPase activity by high Na+ concentrations is indicated semiquantitatively in Table 1. D, K+ dependence of Na+,K+-ATPase activity. Each line shows the best fit of the Hill equation, and the extracted K0.5 values are listed in Table 1. E, distribution of the phosphoenzyme between E1P and E2P at 150 mm NaCl. Each line shows the best fit of a biexponential decay function. The initial amounts of E2P, which correspond to the amplitude of the slow phase, are listed in Table 1. A–E, experimental conditions and equations used for data fitting are described under “Experimental Procedures.” Statistical information is given in Table 1. Symbol and error bars (seen only when larger than the size of the symbols) represent mean ± S.E. Dotted lines reproduce the wild type for direct comparison in the same panel.