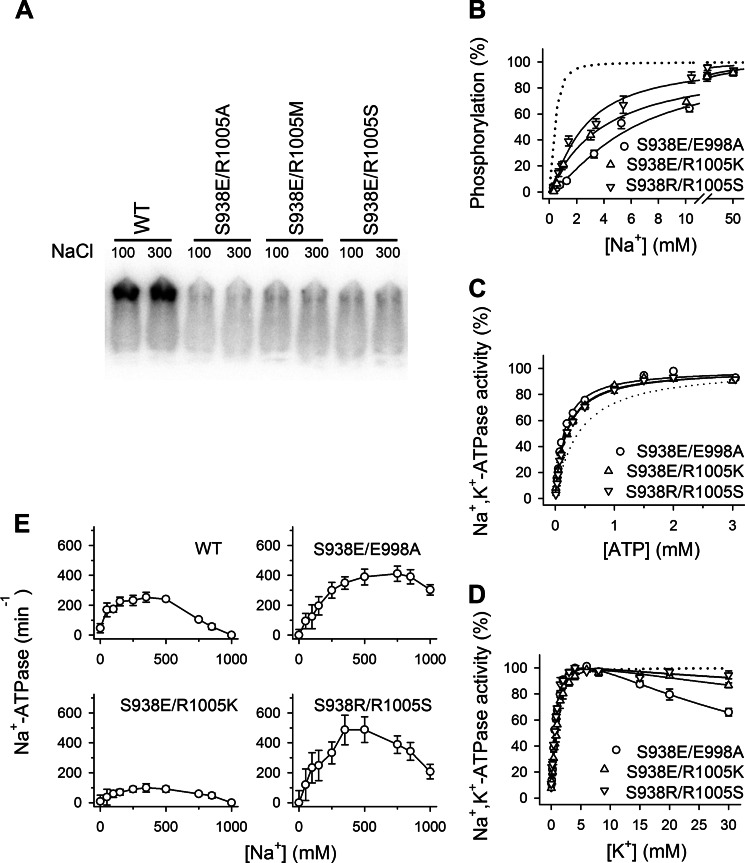
FIGURE 5.
The phosphomimetic mutation S938E mediates its effect through interaction with Arg-1005. A, representative phosphorimaging autoradiograph of transiently expressed double mutants. The double mutants S938E/R1005A, S938E/R1005M, and S938E/R1005S, which could not sustain cell viability in the presence of ouabain, were transiently expressed in the presence of siRNA-targeted knockdown of the endogenous COS-1 Na+,K+-ATPase. The autoradiograph shows 32P incorporation from [γ-32P]ATP at the catalytic site aspartate in the P-domain following separation by SDS-PAGE of enzyme phosphorylated in the presence of the Na+ concentrations indicated in mm. It can be seen that all the double mutants tested were phosphorylation-inactive. B, Na+ dependence of phosphorylation from [γ-32P]ATP. Each line shows the best fit of the Hill equation, and the extracted K0.5 values are listed in Table 1. C, ATP dependence of Na+,K+-ATPase activity. Each line shows the best fit of the Hill equation, and the extracted K0.5 values are listed in Table 1. D, K+ dependence of Na+,K+-ATPase activity. Each line shows the best fit of a two-component Hill equation with the inhibition represented by a negative term. K0.5 values for the rising parts of the curves are listed in Table 1. E, Na+ dependence of Na+-ATPase activity in the absence of K+. Inhibition of Na+-ATPase activity by high Na+ concentrations is indicated semiquantitatively in Table 1. B–E, experimental conditions and equations used for data fitting are described under “Experimental Procedures.” Statistical information is given in Table 1. Symbol and error bars (seen only when larger than the size of the symbols) represent mean ± S.E. Dotted lines reproduce the wild type for direct comparison in the same panel.