Abstract
Background:
Operable non-small cell lung cancer (NSCLC) patients whose tumours have spread to regional or central lymph nodes at the time of diagnosis have dismal prognoses compared with those who have limited disease. The current TNM staging system for NSCLC poorly distinguishes patients with lymph-node metastases who will succumb to, and those who will eventually be cured from, their disease. This novel study: (1) evaluates the presence of different subsets of intraepithelial tumour-infiltrating lymphocytes (TILs) in lymph nodes with metastases from NSCLC patients; (2) explores the impact of intraepithelial TILs in lymph nodes on survival; (3) correlates their presence with both intraepithelial and stromal TILs in their corresponding primary tumours.
Methods:
Metastatic lymph-node tissue from 143N+ NSCLC patients was collected and tissue microarrays were constructed. Immunohistochemistry was used to evaluate the presence of intraepithelial CD3+, CD4+, CD8+, CD20+ and CD45RO+ TILs and their impact on survival.
Results:
A high level of intraepithelial CD45RO+ TILs in lymph-node metastases from N+ NSCLC patients was an independent positive prognostic factor for disease-specific survival in all patients (HR=0.58, P=0.029) and in squamous cell carcinoma (HR=0.31, P=0.006), but not in adenocarcinoma patients.
Conclusions:
The presence of intraepithelial CD45RO+ cells in lymph-node metastases from N+ NSCLC patients predicts favourable disease-specific survival and outperforms the established TNM staging system in the SCC subgroup.
Keywords: NSCLC, lung cancer, TNM-I, biomarker, lymph-node metastasis, CD45RO
Non-small cell lung cancer (NSCLC) constitutes one of the most severe forms of cancer with the highest annual death-counts (Siegel et al, 2014). NSCLC is staged according to tumour size (T), nodal involvement (N) and the presence of metastases (M), combined into the American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC)TNM staging system for lung cancer (Goldstraw et al, 2007). The TNM system has hitherto proven to be robust, and remains the best predictor of patient outcomes. Thus, providing the best platform for treatment decision-making in NSCLC. Clinical stage I-IIIA NSCLC patients are considered candidates for surgical resection, although the prognoses vary highly within this cohort (Goldstraw et al, 2007). Stage I patients have limited tumours and no nodal metastases (N0). Stage IIA-IIIA includes patients with T1-T4 tumours. Most, but not all, patients within these stages will be node positive (N+) (Goldstraw et al, 2007). As a consequence, both T2bN0 and T2aN1 tumours are classified as the same pStage although they may represent two different clinical problems: the former with extended local infiltration causing problems in obtaining negative surgical margins and the latter having acquired the ability to enter the lymphovascular system with increased chance of regional or distant metastasis.
Extensive efforts are invested in finding tumour intrinsic biomarkers able to predict: (1) the natural course of NSCLC as a disease and (2) the chance of recurrence once the primary tumour and resectable lymph-node metastases have been removed (Kerr et al, 2014). However, only a few studies have produced reliable prognostic biomarkers able to assist treatment decision-making whereof none have been able to supplement or surpass the TNM staging system regarding NSCLC prognostication in a curative setting (Kerr et al, 2014). Nevertheless, recent advances in tumour immunology have indicated the presence of TILs to have an important role in the regulation and development of malignant neoplasms (Galon et al, 2013; Donnem et al, 2015). This seems to be a universal concept, demonstrated for several types of cancer including NSCLC, breast- and colorectal cancer (Galon et al, 2013). In colorectal cancer, a worldwide task-force has initiated a large-scale study to investigate whether the prognostic impact of an immunoscore holds true in a prospective setting; and in breast cancer, guidelines for the evaluation of TILs have been proposed (Galon et al, 2014; Salgado et al, 2014). Regarding lung cancer, our group is initiating a large national prospective trial and is currently evaluating different TIL subsets for inclusion in an immunoscore for NSCLC (Donnem et al, 2015).
Simplified, TILs can be divided into four subsets, namely, (1) effector cells, (2) memory cells, (3) regulatory cells and (4) other TILs, each subset expressing distinctive surface receptors. Although both intraepithelial and/or stromal CD3+ (pan-lymphocyte marker, Al-Shibli et al, 2010; Schalper et al, 2015), CD8+ (cytotoxic, Al-Shibli et al, 2008; Donnem et al, 2015; Schalper et al, 2015), CD4+ (T helper, Al-Shibli et al, 2008; Schalper et al, 2015) and CD20+ (B cell, Al-Shibli et al, 2008; Schalper et al, 2015) TILs have been established as positive indicators of prognosis in NSCLC, little is known of intraepithelial TILs in metastatic lymph nodes from N+ NSCLC patients. This study (1) evaluates the presence of different subsets of intraepithelial tumour-infiltrating lymphocytes (TILs) in lymph nodes with metastases from NSCLC patients; (2) explores the impact of intraepithelial TILs in lymph nodes on survival; (3) correlates their presence with both intraepithelial and stromal TILs in their corresponding primary tumours.
Materials and methods
Patients and clinical samples
An unselected population of 172 patients with N+ NSCLC resected in 1990–2010 at the University Hospital of North-Norway and Nordland Hospital were included in this study. This represents a subpopulation of a cohort comprising 536 unselected stage IA-IIIA NSCLC patients previously described by our group (Kilvaer et al, 2015). Of these 172 patients, 39 did not have adequate paraffin-embedded tumour specimens from tumour-positive lymph nodes, leaving 143 patients available for analyses.
This report includes follow-up data as of 1 October 2013. The median follow-up of survivors was 71 months (range 34–199).
Tissue micro-array construction and immunohistochemistry
All lymph-node samples were reviewed by an experienced pathologist (SAS or KAS). The most representative area containing tumour tissue was marked on the haemotoxylin and eosin slide and sampled for tissue micro-array (TMA) blocks. The TMAs were assembled using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA). The methodology is well documented (Bremnes, 2002). Two duplicate cores from each patient were included in the TMAs. In case of more than one available positive LN, the one deemed most suitable for TMA by the pathologist was chosen. Multiple 4-μm sections were cut with a Micron micro-tome (HM355S).
The immunohistochemistry (IHC) procedures for the investigated markers are supplied in Supplementary Table S1. Briefly, all assays were performed on the Ventana Discovery Ultra or XT automated immunostainers (Ventana Medical Systems, Tucson, AZ, USA) with different procedures for each marker.
Scoring of IHC
Representative and viable tissue sections were reviewed using a Leica DM 2500 microscope (Leica Microsystems Ltd., CH9435 Heerbrugg, Switzerland). On the basis of initial review, author Al-Saad established a semi-quantitative score for each marker. The TMA slides were scored for CD3+, CD4+, CD8+, CD20+ and CD45RO+ intraepithelial TILs by two of the authors (MRK and EEP or MRK and RJ). Intra-epithelial TILs were defined as TILs clearly interacting with, or surrounded by malignant epithelial cells. A five-category scale with the following levels was used to score the TMA cores according to percentage of positive cells compared with the total number of cells in the intra-epithelial compartment and divided into five groups: 0=<1%, 1=1–5%, 2=6–25%, 3=26–50% and 4=>50%. When assessing a given core, the observers were blinded to each other, clinical variables and outcome.
High expression was defined as ⩾1% positive cells for CD3, CD4 and CD20 and >5% positive cells for CD8 and CD45RO. Cutoffs for CD3, CD4, CD8 and CD20 were chosen based on previously defined cutoffs for TIL expression in the primary tumour of the same cohort. Cutoff for CD45RO was based on a minimal P-value approach.
Statistical methods
All statistical analyses were conducted using RStudio 0.98.486 with R version 3.2.2 (R Core Team, 2014) and libraries ‘survival' (Therneau and Grambsc, 2000), ‘car' (Fox and Weisberg, 2011), ‘ggplot2' (Wickham, 2009), ‘gridExtra' (Auguie, 2012), ‘Hmisc' (Jr FEH and Charles Dupont, 2015) and ‘irr' (Gamer et al, 2012).
The IHC scores from each observer were compared for interobserver reliability using a two-way random effects model with absolute agreement definition and Cohen's kappa-statistics with equal weights. The intraclass correlation coefficient (reliability coefficient) and Cohen's kappa were obtained from these results.
The Chi-square and Fischer's exact tests were used to examine the association between molecular marker expression and clinicopathological variables. Spearman's rank-correlation was used to examine between marker correlations. Owing to the large number of correlation analyses, Bonferroni corrections were conducted for these analyses.
Univariable survival analyses were done using the Kaplan–Meier method. Statistical difference between survival curves was assessed by the log-rank test. Disease-specific survival (DSS) was defined as the time from diagnosis to cancer-related death. Multivariable analysis, using the Cox proportional hazards model, was carried out to assess the independent value of pretreatment variables in the presence of other variables. Only variables with P<0.25 from the univariate analyses, or deemed important, were explored in multivariable analyses.
The significance level used was P<0.05.
Ethical clearance
This study was approved by the Regional Committee for Medical and Health Research Ethics (Northern Norway, UNN: protocol ID: 2011/2503) and the need for patient consent waived. The collection and storing of the clinical database was approved by the National Data Inspection Board. The reporting of clinicopathological variables, survival data and biomarker expressions was conducted in accordance with the REMARK guidelines (McShane et al, 2006).
Results
Clinicopathological variables
Clinicopathological variables are summarised in Table 1. Median age at diagnosis was 66 years. Twenty-seven percent of the patients were female and ninety-two percent had a performance status ⩽1. All patients underwent surgical resection; 52% received a wedge or lobectomy and 48% a pulmonectomy. The histological distribution comprised 91 (53%) squamous cell carcinomas (SCC), 68 (40%) adenocarcinomas (ADC) and 13 (8%) undifferentiated carcinomas (NOS). In all, 27% of the patients received adjuvant radiotherapy alone, 18% chemotherapy alone and 5% both adjuvant radiotherapy and chemotherapy.
Table 1. Clinicopathological variables as predictors of disease-specific survival in LN+ NSCLC patients in the overall cohort and stratified into the SCC and ADC subgroups (univariate analyses, log-rank test, N=172, 91 and 68, respectively).
|
All |
SCC |
ADC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N(%) | 5Y | M | HR (95% CI) | P | N(%) | 5Y | M | HR (95%CI) | P | N(%) | 5Y | M | HR (95%CI) | P | |
| Age | 0.591 | 0.384 | 0.008 | ||||||||||||
| ⩽65 | 80 (47) | 29 | 24 | 1 | 43 (47) | 45 | 33 | 1 | 34 (50) | 5 | 23 | 1 | |||
| >65 | 92 (53) | 34 | 27 | 0.9 (0.62–1.32) | 48 (53) | 33 | 25 | 1.26 (0.74–2.16) | 34 (50) | 40 | 47 | 0.46 (0.25–0.83) | |||
| Gender | 0.280 | 0.967 | 0.122 | ||||||||||||
| Female | 46 (27) | 34 | 44 | 1 | 15 (16) | 43 | 28 | 1 | 25 (37) | 32 | 44 | 1 | |||
| Male | 126 (73) | 30 | 24 | 1.27 (0.84–1.92) | 76 (84) | 39 | 25 | 1.02 (0.5–2.07) | 43 (63) | 17 | 24 | 1.62 (0.9–2.94) | |||
| ECOG | 0.854 | 0.519 | 0.206 | ||||||||||||
| 0 | 97 (56) | 31 | 27 | 1 | 45 (49) | 33 | 21 | 1 | 43 (63) | 29 | 43 | 1 | |||
| 1 | 61 (35) | 34 | 23 | 1.12 (0.74–1.68) | 38 (42) | 44 | 33 | 0.94 (0.54–1.63) | 20 (29) | 14 | 15 | 1.62 (0.82–3.22) | |||
| 2 | 14 (8) | 38 | 25 | 0.97 (0.43–2.18) | 8 (9) | 57 | NA | 0.44 (0.16–1.24) | 5 (7) | 25 | 17 | 2.02 (0.45–9.13) | |||
| Smoking | 0.094 | 0.252 | 0.266 | ||||||||||||
| Never | 5 (3) | 0 | 14 | 1 | 3 (3) | 0 | 12 | 1 | 2 (3) | 0 | 15 | 1 | |||
| Previous | 103 (60) | 31 | 29 | 0.36 (0.07–1.86) | 53 (58) | 39 | 29 | 0.33 (0.03–3.75) | 43 (63) | 18 | 25 | 0.4 (0.04–3.93) | |||
| Present | 64 (37) | 35 | 25 | 0.34 (0.06–1.8) | 35 (38) | 43 | 28 | 0.31 (0.03–3.59) | 23 (34) | 32 | 36 | 0.32 (0.03–3.17) | |||
| Weight loss | 0.477 | 0.593 | 0.410 | ||||||||||||
| <10% | 154 (90) | 32 | 25 | 1 | 81 (89) | 40 | 28 | 1 | 61 (90) | 26 | 27 | 1 | |||
| ⩾10% | 18 (10) | 16 | 15 | 1.25 (0.63–2.48) | 10 (11) | 44 | 11 | 1.28 (0.46–3.56) | 7 (10) | 0 | 15 | 1.43 (0.53–3.84) | |||
| Surgical procedure | 0.326 | 0.970 | 0.116 | ||||||||||||
| Wedge/Lobectomy | 89 (52) | 32 | 41 | 1 | 38 (42) | 41 | 28 | 1 | 45 (66) | 26 | 43 | 1 | |||
| Pulmonectomy | 83 (48) | 31 | 20 | 1.21 (0.82–1.78) | 53 (58) | 38 | 21 | 1.01 (0.59–1.73) | 23 (34) | 20 | 15 | 1.62 (0.83–3.19) | |||
| Margins | 0.195 | 0.364 | 0.626 | ||||||||||||
| Free | 148 (86) | 34 | 27 | 1 | 77 (85) | 42 | 28 | 1 | 59 (87) | 25 | 27 | 1 | |||
| Not free | 24 (14) | 16 | 23 | 1.41 (0.78–2.53) | 14 (15) | 26 | 25 | 1.37 (0.64–2.94) | 9 (13) | 0 | 23 | 1.24 (0.49–3.15) | |||
| T stage | 0.009 | 0.026 | 0.018 | ||||||||||||
| IA | 12 (7) | 89 | 127 | 1 | 6 (7) | 100 | 127 | 1 | 6 (9) | 83 | NA | 1 | |||
| IB | 21 (12) | 25 | 28 | 6.43 (3–13.76) | 9 (10) | 44 | 33 | 4.95 (1.64–14.87) | 9 (13) | 0 | 24 | 9.99 (3.26–30.62) | |||
| IIA | 69 (40) | 34 | 25 | 6.58 (3.56–12.15) | 36 (40) | 42 | 29 | 6.22 (2.58–15) | 30 (44) | 23 | 24 | 8.14 (3.49–18.98) | |||
| IIB | 29 (17) | 21 | 20 | 7.99 (3.84–16.62) | 18 (20) | 21 | 16 | 9.5 (3.4–26.52) | 10 (15) | 22 | 47 | 5.28 (1.88–14.8) | |||
| III | 37 (22) | 18 | 16 | 9.1 (4.45–18.61) | 19 (21) | 33 | 17 | 7.06 (2.55–19.56) | 13 (19) | 0 | 12 | 14.51 (4.71–44.69) | |||
| IV | 4 (2) | 0 | 15 | 15.61 (2.52–96.52) | 3 (3) | 0 | 10 | 27.6 (1.37–556) | |||||||
| N stage | 0.035 | 0.010 | 0.542 | ||||||||||||
| 1 | 118 (69) | 36 | 35 | 1 | 73 (80) | 45 | 35 | 1 | 39 (57) | 25 | 30 | 1 | |||
| 2 | 54 (31) | 21 | 19 | 1.52 (0.99–2.34) | 18 (20) | 18 | 13 | 2.14 (1.02–4.51) | 29 (43) | 23 | 24 | 1.2 (0.65–2.22) | |||
| P stage | 0.016 | 0.035 | 0.416 | ||||||||||||
| IIA | 72 (42) | 44 | 43 | 1 | 43 (47) | 55 | 71 | 1 | 26 (38) | 28 | 30 | 1 | |||
| IIB | 16 (9) | 32 | 28 | 1.27 (0.66–2.46) | 12 (13) | 31 | 19 | 1.59 (0.7–3.61) | 4 (6) | 38 | 47 | 0.67 (0.21–2.09) | |||
| IIIA | 84 (49) | 19 | 17 | 1.79 (1.19–2.68) | 36 (40) | 23 | 15 | 2.09 (1.15–3.83) | 38 (56) | 11 | 24 | 1.37 (0.74–2.53) | |||
| Histology | 0.869 | ||||||||||||||
| SCC | 91 (53) | 39 | 25 | 1 | |||||||||||
| ADC | 68 (40) | 23 | 25 | 1.1 (0.74–1.65) | |||||||||||
| NOS | 13 (8) | 17 | 19 | 1.12 (0.52–2.41) | |||||||||||
| Differentiation | 0.417 | 0.399 | 0.851 | ||||||||||||
| Poor | 94 (55) | 25 | 24 | 1 | 41 (45) | 32 | 20 | 1 | 40 (59) | 19 | 27 | 1 | |||
| Moderate | 68 (40) | 38 | 33 | 0.84 (0.57–1.25) | 45 (49) | 46 | 35 | 0.75 (0.43–1.31) | 23 (34) | 24 | 21 | 0.96 (0.51–1.81) | |||
| Well | 10 (6) | 43 | 41 | 0.56 (0.25–1.26) | 5 (5) | 38 | 41 | 0.47 (0.15–1.43) | 5 (7) | 60 | NA | 0.66 (0.2–2.22) | |||
| Vascular infiltration | 0.018 | 0.007 | 0.808 | ||||||||||||
| No | 128 (74) | 37 | 30 | 1 | 68 (75) | 46 | 35 | 1 | 54 (79) | 22 | 24 | 1 | |||
| Yes | 43 (25) | 18 | 18 | 1.65 (1.02–2.68) | 23 (25) | 17 | 18 | 2.16 (1.05–4.44) | 13 (19) | 30 | 36 | 0.91 (0.43–1.92) | |||
| Missing | 1 (1) | 1 (1) | |||||||||||||
Abbreviations: ADC=adenocarcinom; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; LN, lymph node; NOS=not otherwise specified; NSCLC= non-small cell lung cancer; SCC=squamous cell carcinoma.
Expression of CD3+, CD4+, CD8+, CD20+ and CD45RO+ cells in resected lymph nodes of LN+ NSCLC patients, their correlations and inter-observer variability
In all observed cores, the malignant epithelial cells either dominated the entire core or grew in a lens shape from the capsule of the LN. Intraepithelial TILs were distinguished from normal lymphocytes based on histological evaluation of spatial location of the TILs. A minority of LN metastases incorporated stromal areas comparable to the original tumour. Unfortunately, it was not possible to determine where this stromal area stopped and the normal lymphatic tissue started using the available methods. Hence, this finding was not explored further in the current study. CD3, CD4, CD8, CD20 and CD45RO were expressed on the surface and in the cytoplasm of immune cells (Supplementary Figure S1). Between-scorer agreement was excellent with ICC>0.80 for all markers (Supplementary Table S2).
After Bonferroni correction, no significant associations between intraepithelial TILs and clinicopathological variables were discovered (Supplementary Table S3). There were extensive correlations between CD3+, CD4+, CD8+, CD20+ and CD45RO+ intraepithelial TILs in lymph-node metastases while no significant correlations to their stromal and intraepithelial counterparts in the primary tumours were observed (Supplementary Table S4).
Univariable analyses
Table 1 summarises the clinicopathological variables and their impact on disease-specific survival (DSS). Increasing tStage, nStage, pStage and the presence of vascular infiltration were significant negative prognostic indicators of DSS in the overall cohort and in the SCC subgroup. Only tStage and age ⩽65 were significant negative prognostic factors in the ADC subgroup.
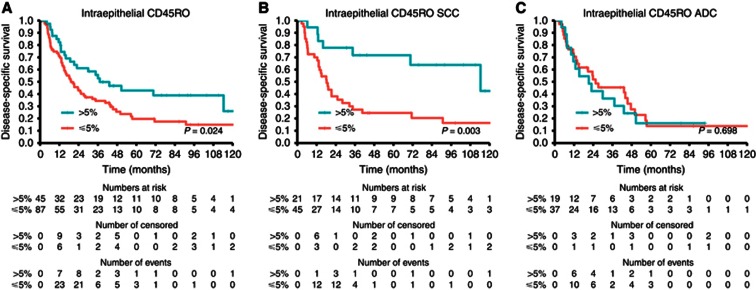
Table 2A and Supplementary Figure S2 summarise the investigated markers, their impact on DSS in the overall cohort and in the SCC and ADC subgroups. The presence of CD45RO+ TILs was a significant positive prognostic factor in the overall cohort (HR=0.58, P=0.024) and in the SCC subgroup (HR=0.31, P=0.003), but not in the ADC subgroup (Figure 1).
Table 2. (A) Intraepithelial expression of CD3, CD4, CD8, CD20 and CD45RO in resected lymph nodes of LN+ NSCLC patients as predictors of disease-specific survival in the overall cohort and stratified into the SCC and ADC subgroups. (B) Multivariable models summarising significant independent prognostic factors in the total cohort and in the SCC subgroup (univariate analyses, log-rank test and Cox regression analyses, N=172, 91 and 68, respectively).
|
All |
SCC |
ADC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 5Y | M | HR (95%CI) | P | N (%) | 5Y | M | HR (95%CI) | P | N (%) | 5Y | M | HR (95%CI) | P | |
| A | |||||||||||||||
| CD3 | 0.875 | 0.794 | 0.608 | ||||||||||||
| <1% | 14 (8) | 30 | 29 | 1 | 9 (10) | 43 | 33 | 1 | 5 (7) | 0 | 23 | 1 | |||
| ⩾1% | 113 (66) | 30 | 24 | 1.06 (0.54–2.09) | 56 (62) | 44 | 35 | 1.13 (0.46–2.8) | 47 (69) | 17 | 24 | 0.77 (0.24–2.43) | |||
| Missing | 45 (26) | 26 (29) | 16 (24) | ||||||||||||
| CD4 | 0.209 | 0.740 | 0.097 | ||||||||||||
| <1% | 54 (31) | 39 | 25 | 1 | 29 (32) | 47 | 25 | 1 | 23 (34) | 30 | 25 | 1 | |||
| ⩾1% | 63 (37) | 21 | 27 | 1.34 (0.84–2.14) | 29 (32) | 42 | 35 | 1.12 (0.55–2.28) | 29 (43) | 0 | 21 | 1.73 (0.91–3.29) | |||
| Missing | 55 (32) | 33 (36) | 16 (24) | ||||||||||||
| CD8 | 0.277 | 0.321 | 0.410 | ||||||||||||
| ⩽5% | 37 (22) | 18 | 23 | 1 | 21 (23) | 33 | 25 | 1 | 14 (21) | 0 | 23 | 1 | |||
| >5% | 88 (51) | 33 | 21 | 0.77 (0.47–1.27) | 43 (47) | 45 | 27 | 0.71 (0.35–1.46) | 37 (54) | 21 | 21 | 0.75 (0.36–1.56) | |||
| Missing | 47 (27) | 27 (30) | 17 (25) | ||||||||||||
| CD20 | 0.681 | 0.312 | 0.954 | ||||||||||||
| <1% | 40 (23) | 29 | 25 | 1 | 21 (23) | 34 | 29 | 1 | 17 (25) | 19 | 15 | 1 | |||
| ⩾1% | 82 (48) | 30 | 24 | 0.91 (0.57–1.46) | 39 (43) | 46 | 21 | 0.71 (0.36–1.41) | 35 (51) | 16 | 24 | 1.02 (0.5–2.07) | |||
| Missing | 50 (29) | 31 (34) | 16 (24) | ||||||||||||
| CD45RO | 0.024 | 0.003 | 0.698 | ||||||||||||
| ⩽5% | 87 (51) | 20 | 19 | 1 | 45 (49) | 25 | 18 | 1 | 37 (54) | 14 | 25 | 1 | |||
| >5% | 45 (26) | 43 | 37 | 0.58 (0.37–0.9) | 21 (23) | 72 | 114 | 0.31 (0.16–0.6) | 19 (28) | 16 | 21 | 1.14 (0.58–2.22) | |||
| Missing | 40 (23) | 25 (27) | 12 (18) | ||||||||||||
| B | |||||||||||||||
| CD45RO | |||||||||||||||
| ⩽5% | 1 | 1 | |||||||||||||
| >5% | 0.58 (0.35–0.95) | 0.029 | 0.31 (0.14–0.71) | 0.006 | |||||||||||
| T stage | |||||||||||||||
| IA | 1 | ||||||||||||||
| IB | 7.42 (0.96–57.3) | 0.055 | |||||||||||||
| IIA | 8.7 (1.19–63.64) | 0.033 | |||||||||||||
| IIB | 7.12 (0.93–54.65) | 0.059 | |||||||||||||
| III | 9.89 (1.32–74.01) | 0.026 | |||||||||||||
| IV | 20.26 (2.09–196.9) | 0.010 | |||||||||||||
| Vascular infiltration | |||||||||||||||
| No | 1 | ||||||||||||||
| Yes | 1.92 (0.96–3.82) | 0.065 | |||||||||||||
Abbreviations: ADC=adenocarcinom; CD=cluster of differentiation; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; LN=lymph node; NOS=not otherwise specified; NSCLC= non-small cell lung cancer; SCC=squamous cell carcinoma.
Figure 1.
Disease-specific survival curves for (A) CD45RO in the overall cohort; (B) CD45RO in SCC; (C) CD45RO in ADC. Abbreviations: SCC=squamous cell carcinoma; ADC=adenocarcinoma.
Multivariable analyses
Table 2B summarises the multivariable models for DSS in the overall cohort and in the SCC subgroup. The presence of CD45RO+ cells (HR=0.58, P=0.029) and tStage were significant independent indicators of DSS in the overall cohort, while only the presence of CD45RO+ cells (HR=0.31, P=0.006) was a significant independent indicator of DSS in the SCC subgroup.
Discussion
The lymphatic system comprises a network of ducts and nodules able to drain excess fluid and waste products due to cellular breakdown, inflammation or infection from the peripheral tissues (Alitalo, 2011). As a consequence, the lymphatic fluid filtered through secondary lymphoid organs (SLOs), such as lymph nodes, is rich in potential antigens which is why adaptive immune responses are initiated here (Alitalo, 2011). Recent discoveries and novel treatment strategies have emphasised the adaptive immune-system's role in cancer development and control (Schreiber et al, 2011; Galon et al, 2013). Strong evidence support a theory of a multi-step interaction between the immune-system and initiation of cancer, leading to (1) elimination of cancer cells, (2) equilibrium and containment of the developing cancer or (3) cancer cells' evasion of the immune-system and subsequent clinical cancer with the potential of locally advanced disease or distant metastases (Schreiber et al, 2011; Galon et al, 2013). In metastatic NSCLC, therapy with immune-checkpoint inhibitors has recently proven a feasible option, with durable responses seen in some patients (Borghaei et al, 2015; Brahmer et al, 2015; Garon et al, 2015). These studies provide circumstantial evidence for an ongoing, although in-efficient, immune response in the metastatic sites, which can be further exploited. What initiates, drives and controls these responses remains largely unknown, but immune cells are undoubtedly involved. We hypothesised that immune cell infiltration into lymph-node metastases would prove prognostic in N+ NSCLC patients.
NSCLC patients presenting with lymph-node metastases comprise a group whose expected outcome is adverse compared with those with N0 disease. This is reflected in the AJCC/UICC TNM staging where N+ translates into more advanced stage and worse survival compared with N0 (Goldstraw et al, 2007). If assessed as resectable, then patients with stage II-IIIA disease undergo treatment with curative intent, though the majority eventually succumb to their disease. In this study, we observe that intraepithelial CD45RO+, and not CD3+, CD4+, CD8+ or CD20+, TILs in the metastatic lymph nodes, represent an independent positive prognostic factor in this patient group.
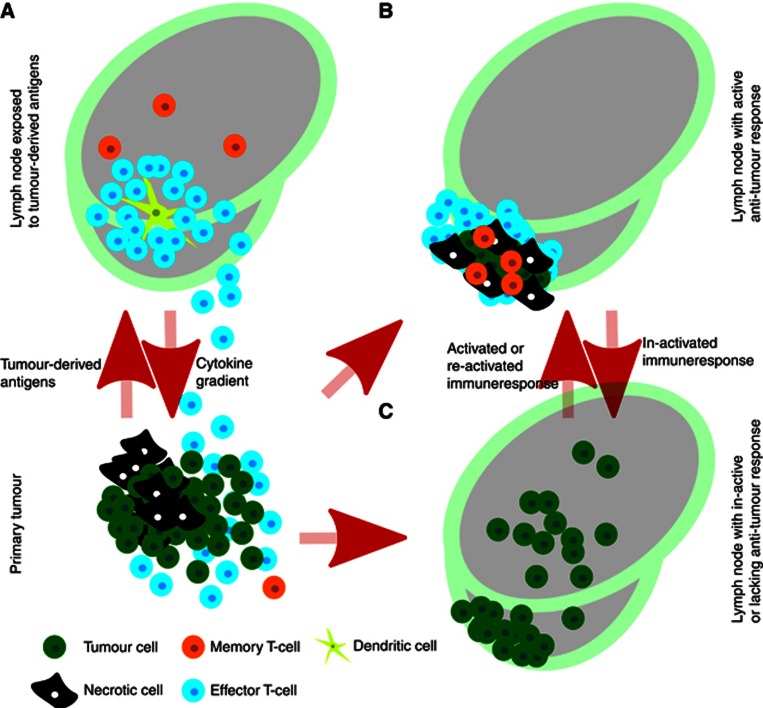
With the exception of T memory-stem cells, CD45RO is generally considered to mark all subsets of memory T-cells, including those of the bone marrow and SLOs, circulating and tissue-resident subtypes (Farber et al, 2013). Memory T cells are known to undergo proliferative expansion upon re-stimulation with antigen and thus soliciting a robust immune response (Farber et al, 2013). Whether memory T cells develop and become activated in the NSCLC patient's lymph nodes, travel from the primary tumours or derive from the tertiary lymphoid structures (TLS), remains unknown. They may arise in the lymph nodes before the arrival of tumour cells due to exposure to tumour antigens from apoptotic or necrotic tumour cells (Figure 2A) or they may form after the arrival of tumour cells (Figures 2B and C). It would be highly interesting to further elucidate these mechanisms through studies in patient sentinel node biopsies.
Figure 2.
During tumour development some tumour cells will eventually die, either through apoptosis or necrosis, and provide degradation products that may be picked up by antigen-presenting cells such as dendritic cells (DCs). DCs may subsequently present the antigens to T cells and potentially elicit an immune response (A). Once an immune response is initiated, a subset of T cells might differentiate into memory T cells (A and B). When presented to antigen, memory T cells have the potential to quickly respond, proliferate and attack their target eliciting a strong immune response controlling and possibly eradicating the metastatic cancer cells (B). In the absence of a durable immune response, either due to lack of antigen-specific T cells, tumour cell immune evasion or immunoediting, tumour cells spreading to the lymph nodes are free to multiply and form secondary distant organ metastases (C).
While 26% and 51% of patients in the CD45RO- group succumb to their disease within the first 12 and 24 months after diagnosis, this was seen in only 16% and 33% in CD45RO+ group. In the SCC subgroup, this was more pronounced, with 27% and 56% and 5% and 19% lung cancer-specific deaths after 12 and 24 months in the CD45RO- and CD45RO+ groups, respectively. Experiments show naive T cells may survive for several years while memory T cells have a half-life of 1–12 months (Farber et al, 2013). Our results may indicate that, although present at the time of diagnosis, memory T cells and subsequently the immune response fail for a subgroup of patients. Whether this is the effect of memory T-cell depletion and/or abrogated immune responses due to immune evasion, immunoediting or some other mechanism(s), remains unknown. Nevertheless, we may speculate that for this subgroup of patients, peripheral working checkpoint inhibitors, such as anti-PD-1 or PD-L1 may constitute a promising treatment, as the patients harbour an immune capacity that may be revived. Another potential clinical application lies in choosing which patients should undergo surgery. This has to be elucidated through further prospective trials utilising the expression of CD45RO in LN as a prognosticator. A starting point could be to select stage IIIB patients and stage IIIA patients with borderline resectable disease, that otherwise would receive palliative radiochemotherapy, for potential radical/curative treatment. Clearly, this approach presupposes LNs availability for biopsies. However, before any clinical implementations of the presented results are considered, studies to confirm both inter- and intra-patient PPV and NPV of CD45RO+ TILs in LNs have to be instigated.
Owing to the current understanding of the adaptive immune response (Alitalo, 2011), a close correlation between intraepithelial and stromal immune infiltrates in both primary tumours and metastatic lymph nodes was anticipated. In this study, however, no such relationships were observed, perhaps indicating a distinction between immune responses taking place in the primary tumour vs metastatic lymph nodes. During the last decade TLSs have gained momentum as a provider of anti-tumour immune response (Goc et al, 2014). As NSCLC is associated with TLSs formation (Dieu-Nosjean et al, 2008), the lack of correlation between TILs in the primary tumour and metastatic lymph nodes suggests that the primary adaptive immune response in NSCLC patients form in TLSs. These results may shed light on why reliable biomarkers for the efficacy of immune check-point inhibitors in NSCLC have proven difficult to establish, as efforts have focused on biopsies from the primary tumours and not the metastatic sites (Borghaei et al, 2015; Garon et al, 2015).
Conclusions
The presence of intraepithelial CD45RO+ TILs in lymph-node metastases is a good candidate marker for an immunoscore in SCC N+ NSCLC patients, for which the current TNM staging system is lacking a real prognostic value. In addition, the presence of intraepithelial CD45RO+ TILs may predict the SCC N+ NSCLC patients most likely to benefit from adjuvant cancer immunotherapy.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Alitalo K (2011) The lymphatic vasculature in disease. Nat Med 17: 1371–1380. [DOI] [PubMed] [Google Scholar]
- Al-Shibli K, Al-Saad S, Andersen S, Donnem T, Bremnes RM, Busund L-T (2010) The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. APMIS 118: 371–382. [DOI] [PubMed] [Google Scholar]
- Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T (2008) Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14: 5220–5227. [DOI] [PubMed] [Google Scholar]
- Auguie B (2012) gridExtra: functions in Grid graphics. Available from http://cran.r-project.org/package=gridExtra.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 1–13. [DOI] [PMC free article] [PubMed]
- Bremnes RM (2002) High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol 20: 2417–2428. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman W-H, Cadranel J (2008) Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 26: 4410–4417. [DOI] [PubMed] [Google Scholar]
- Donnem T, Hald SM, Paulsen E-E, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M, Poehl M, Olsen KE, Ditzel HJ, Hansen O, Al-Shibli KI, Kiselev Y, Sandanger TM, Andersen S, Pezzella F, Bremnes RM, Busund L-TR (2015) Stromal CD8+ T cell density - a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res 21: 2635–2643. [DOI] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, Restifo NP (2013) Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol 14: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S (2011) An {R} Companion to Applied Regression. Thousand Oaks, CA, USA: Sage. [Google Scholar]
- Galon J, Angell HK, Bedognetti D, Marincola FM (2013) The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 39: 11–26. [DOI] [PubMed] [Google Scholar]
- Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D'Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope Fa, Scripcariu V, Ascierto Pa, Marincola FM, Fox Ba, Pagès F (2014) Towards the introduction of the ‘Immunoscore' in the classification of malignant tumours. J Pathol 232: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Lemon J, Ian Fellows PS (2012) irr: Various Coefficients of Interrater Reliability and Agreement. Available from http://cran.r-project.org/package=irr.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn M, Felip E, Lee J, Hellmann MD, Hamid O, Goldman JW, Soria J, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018–2028.25891174 [Google Scholar]
- Goc J, Fridman W-H, Hammond SA, Sautès-Fridman C, Dieu-Nosjean M-C (2014) Tertiary lymphoid structures in human lung cancers, a new driver of antitumor immune responses. Oncoimmunology 3: e28976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee, Participating Institutions (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2: 706–714. [DOI] [PubMed] [Google Scholar]
- Jr FEHCharles Dupont (2015) Hmisc: Harrell Miscellaneous. Available from http://cran.r-project.org/package=Hmisc.
- Kerr KM, Bubendorf L, Edelman MJ, Marchetti A, Mok T, Novello S, O'Byrne K, Stahel R, Peters S, Felip E (2014) Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 25: 1462–1474. [DOI] [PubMed] [Google Scholar]
- Kilvaer TK, Khanehkenari MR, Hellevik T, Al-Saad S, Paulsen E-E, Bremnes RM, Busund L-T, Donnem T, Martinez IZ (2015) Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS One 10: e0134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100: 229–235. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing (Vienna, Austria). Available from http://www.r-project.org/.
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez Ea, Thompson Ea, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S (2014) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL (2015) Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 107: dju435–dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsc PM (2000) Modeling Survival Data: Extending the Cox Model. Springer: New York. [Google Scholar]
- Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer: New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.