Abstract
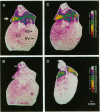
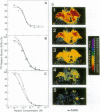
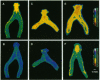
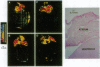
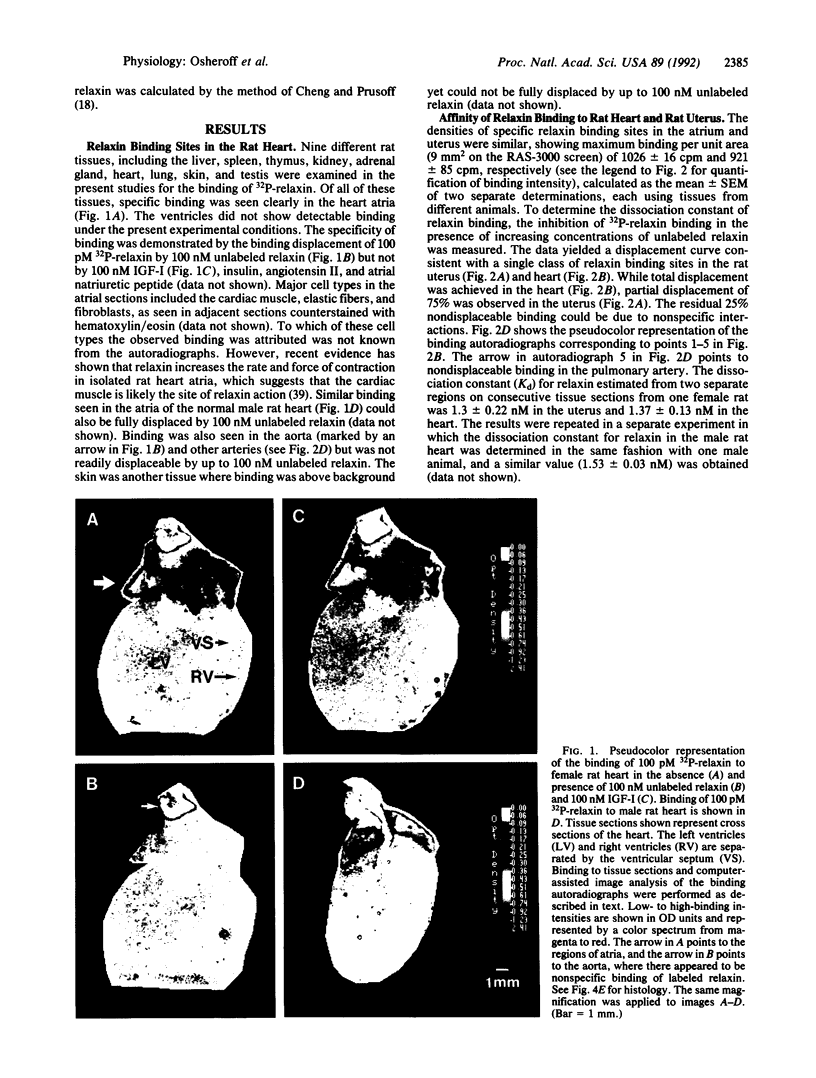
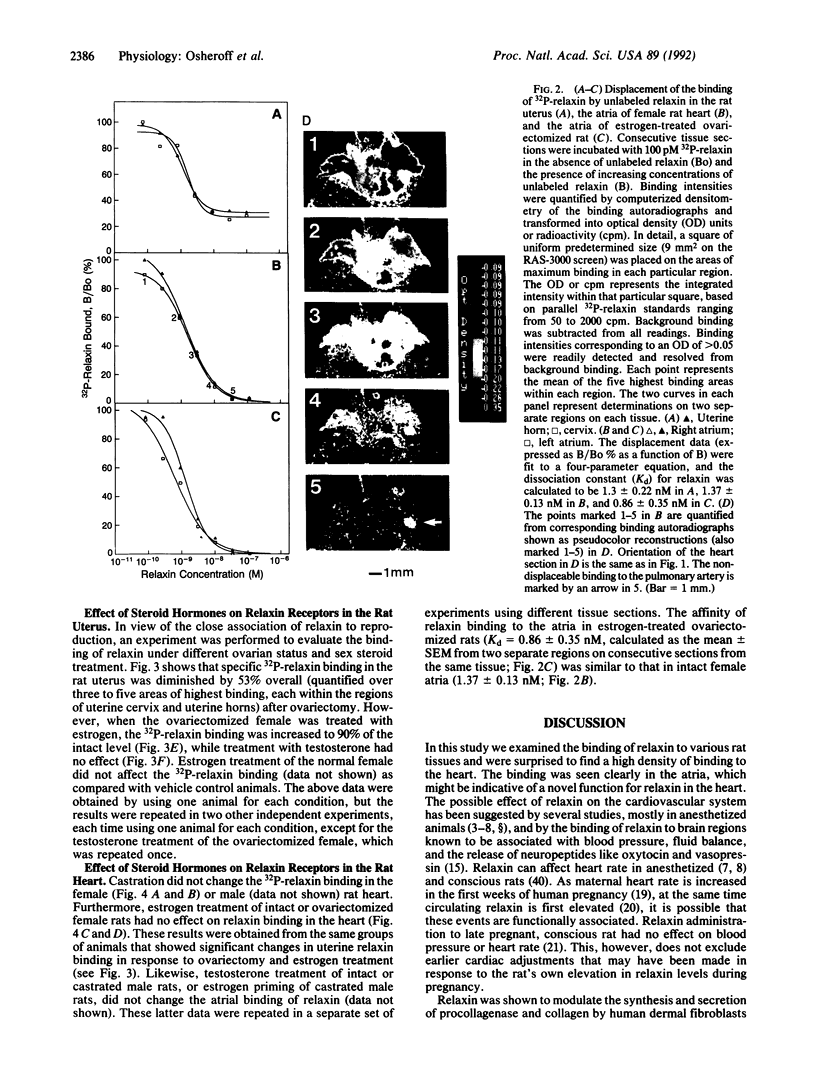
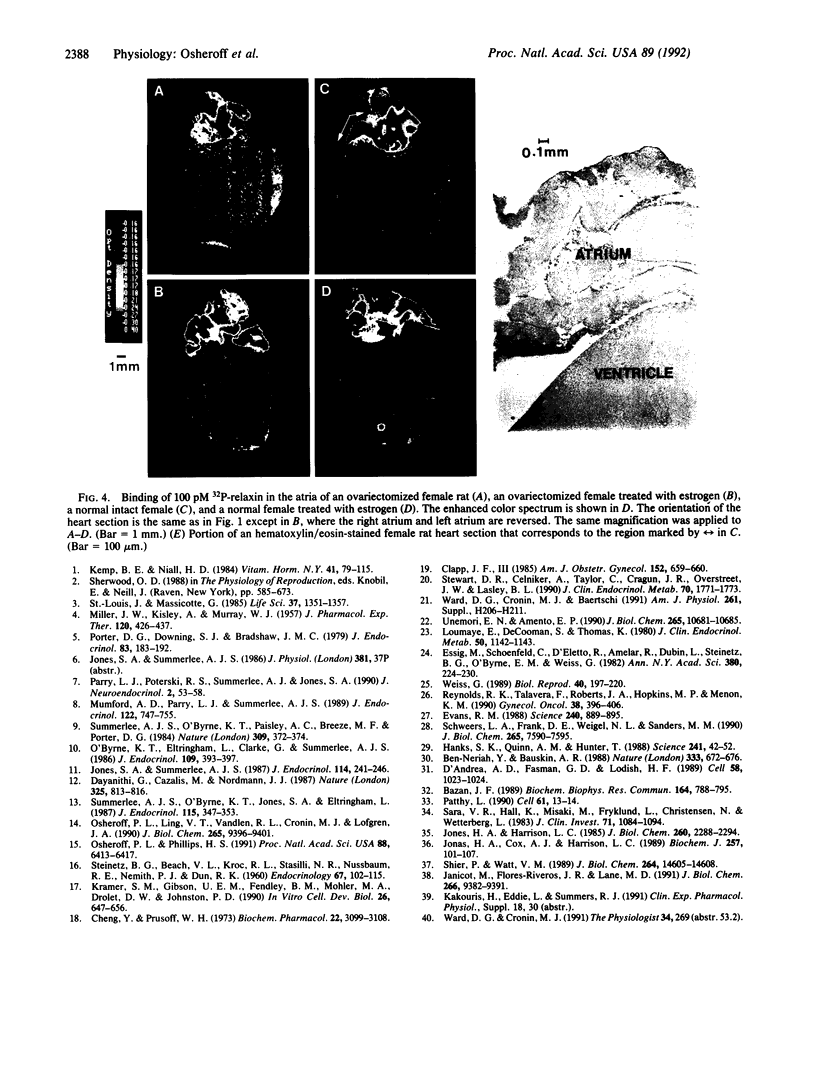
Relaxin is a member of the insulin family of polypeptides that is best known as a reproductive hormone. In an effort to elucidate the mechanism of action of relaxin we previously localized the specific binding sites of a 32P-labeled relaxin in the rat uterus and brain. These studies suggested that, in addition to its classical role in pregnancy, relaxin might have other physiological functions. In the present paper we describe the specific and high-affinity binding of relaxin to the cardiac atrium of both male and female rats. The relaxin binding could not be displaced by peptides belonging to the same family [insulin, insulin-like growth factor I (IGF-I)] or by peptides that were identified in the atrium or were known to have cardiovascular functions (atrial natriuretic peptide, angiotensin II). The dissociation constant for relaxin in the atrium was estimated to be 1.4 nM, which was similar to that found in the uterus (1.3 nM) and the brain (1.4 nM). In view of the close association of relaxin with reproduction, an experiment was also performed to compare the relaxin binding in the uterus and heart after gonadectomy and sex steroid treatment. It was found that the relaxin binding in the rat uterus was diminished by 53% overall following ovariectomy but was restored to 90% of normal levels when treated with estrogen (but not with testosterone). In contrast, the relaxin binding in the rat heart was not affected by castration or sex steroid treatment. We conclude that specific and high-affinity relaxin receptors exist in the atrium of both the male and female rat heart and that these are regulated differently than the relaxin receptors in the uterus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bauskin A. R. Leukocytes express a novel gene encoding a putative transmembrane protein-kinase devoid of an extracellular domain. Nature. 1988 Jun 16;333(6174):672–676. doi: 10.1038/333672a0. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Clapp J. F., 3rd Maternal heart rate in pregnancy. Am J Obstet Gynecol. 1985 Jul 15;152(6 Pt 1):659–660. doi: 10.1016/s0002-9378(85)80040-5. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- Dayanithi G., Cazalis M., Nordmann J. J. Relaxin affects the release of oxytocin and vasopressin from the neurohypophysis. 1987 Feb 26-Mar 4Nature. 325(6107):813–816. doi: 10.1038/325813a0. [DOI] [PubMed] [Google Scholar]
- Essig M., Schoenfeld C., D'Eletto R. T., Amelar R., Steinetz B. G., O'Byrne E. M., Weiss G. Relaxin in human seminal plasma. Ann N Y Acad Sci. 1982;380:224–230. doi: 10.1111/j.1749-6632.1982.tb18045.x. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Janicot M., Flores-Riveros J. R., Lane M. D. The insulin-like growth factor 1 (IGF-1) receptor is responsible for mediating the effects of insulin, IGF-1, and IGF-2 in Xenopus laevis oocytes. J Biol Chem. 1991 May 25;266(15):9382–9391. [PubMed] [Google Scholar]
- Jonas H. A., Cox A. J., Harrison L. C. Delineation of atypical insulin receptors from classical insulin and type I insulin-like growth factor receptors in human placenta. Biochem J. 1989 Jan 1;257(1):101–107. doi: 10.1042/bj2570101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas H. A., Harrison L. C. The human placenta contains two distinct binding and immunoreactive species of insulin-like growth factor-I receptors. J Biol Chem. 1985 Feb 25;260(4):2288–2294. [PubMed] [Google Scholar]
- Jones S. A., Summerlee A. J. Effects of chronic infusion of porcine relaxin on oxytocin release in lactating rats. J Endocrinol. 1987 Aug;114(2):241–246. doi: 10.1677/joe.0.1140241. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Niall H. D. Relaxin. Vitam Horm. 1984;41:79–115. doi: 10.1016/s0083-6729(08)60088-6. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Gibson U. E., Fendly B. M., Mohler M. A., Drolet D. W., Johnston P. D. Increase in cyclic AMP levels by relaxin in newborn rhesus monkey uterus cell culture. In Vitro Cell Dev Biol. 1990 Jun;26(6):647–656. doi: 10.1007/BF02624216. [DOI] [PubMed] [Google Scholar]
- Loumaye E., De Cooman S., Thomas K. Immunoreactive relaxin-like substance in human seminal plasma. J Clin Endocrinol Metab. 1980 Jun;50(6):1142–1143. doi: 10.1210/jcem-50-6-1142. [DOI] [PubMed] [Google Scholar]
- MILLER J. W., KISLEY A., MURRAY W. J. The effects of relaxin-containing ovarian extracts on various types of smooth muscle. J Pharmacol Exp Ther. 1957 Aug;120(4):426–437. [PubMed] [Google Scholar]
- Mumford A. D., Parry L. J., Summerlee A. J. Lesion of the subfornical organ affects the haemotensive response to centrally administered relaxin in anaesthetized rats. J Endocrinol. 1989 Sep;122(3):747–755. doi: 10.1677/joe.0.1220747. [DOI] [PubMed] [Google Scholar]
- O'Byrne K. T., Eltringham L., Clarke G., Summerlee A. J. Effects of porcine relaxin on oxytocin release from the neurohypophysis in the anaesthetized lactating rat. J Endocrinol. 1986 Jun;109(3):393–397. doi: 10.1677/joe.0.1090393. [DOI] [PubMed] [Google Scholar]
- Osheroff P. L., Ling V. T., Vandlen R. L., Cronin M. J., Lofgren J. A. Preparation of biologically active 32P-labeled human relaxin. Displaceable binding to rat uterus, cervix, and brain. J Biol Chem. 1990 Jun 5;265(16):9396–9401. [PubMed] [Google Scholar]
- Osheroff P. L., Phillips H. S. Autoradiographic localization of relaxin binding sites in rat brain. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6413–6417. doi: 10.1073/pnas.88.15.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Porter D. G., Downing S. J., Bradshaw J. M. Relaxin inhibits spontaneous and prostaglandin-driven myometrial activity in anaesthetized rats. J Endocrinol. 1979 Nov;83(2):183–192. doi: 10.1677/joe.0.0830183. [DOI] [PubMed] [Google Scholar]
- Reynolds R. K., Talavera F., Roberts J. A., Hopkins M. P., Menon K. M. Regulation of epidermal growth factor and insulin-like growth factor I receptors by estradiol and progesterone in normal and neoplastic endometrial cell cultures. Gynecol Oncol. 1990 Sep;38(3):396–406. doi: 10.1016/0090-8258(90)90081-u. [DOI] [PubMed] [Google Scholar]
- STEINETZ B. G., BEACH V. L., KROC R. L., STASILLI N. R., NUSSBAUM R. E., NEMITH P. J., DUN R. K. Bioassay of relaxin using a reference standard: a simple and reliable method utilizing direct measurement of interpubic ligament formation in mice. Endocrinology. 1960 Jul;67:102–115. doi: 10.1210/endo-67-1-102. [DOI] [PubMed] [Google Scholar]
- Sara V. R., Hall K., Misaki M., Fryklund L., Christensen N., Wetterberg L. Ontogenesis of somatomedin and insulin receptors in the human fetus. J Clin Invest. 1983 May;71(5):1084–1094. doi: 10.1172/JCI110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers L. A., Frank D. E., Weigel N. L., Sanders M. M. The steroid-dependent regulatory element in the ovalbumin gene does not function as a typical steroid response element. J Biol Chem. 1990 May 5;265(13):7590–7595. [PubMed] [Google Scholar]
- Shier P., Watt V. M. Primary structure of a putative receptor for a ligand of the insulin family. J Biol Chem. 1989 Sep 5;264(25):14605–14608. [PubMed] [Google Scholar]
- St-Louis J., Massicotte G. Chronic decrease of blood pressure by rat relaxin in spontaneously hypertensive rats. Life Sci. 1985 Oct 7;37(14):1351–1357. doi: 10.1016/0024-3205(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Stewart D. R., Celniker A. C., Taylor C. A., Jr, Cragun J. R., Overstreet J. W., Lasley B. L. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990 Jun;70(6):1771–1773. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., O'Byrne K. T., Jones S. A., Eltringham L. The subfornical organ and relaxin-induced inhibition of reflex milk ejection in lactating rats. J Endocrinol. 1987 Nov;115(2):347–353. doi: 10.1677/joe.0.1150347. [DOI] [PubMed] [Google Scholar]
- Summerlee A. J., O'Byrne K. T., Paisley A. C., Breeze M. F., Porter D. G. Relaxin affects the central control of oxytocin release. Nature. 1984 May 24;309(5966):372–374. doi: 10.1038/309372a0. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Amento E. P. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990 Jun 25;265(18):10681–10685. [PubMed] [Google Scholar]
- Ward D. G., Cronin M. J., Baertschi A. J. Lack of cardiovascular and vasopressin responses to human relaxin in conscious, late-pregnant rats. Am J Physiol. 1991 Jul;261(1 Pt 2):H206–H211. doi: 10.1152/ajpheart.1991.261.1.H206. [DOI] [PubMed] [Google Scholar]
- Weiss G. Relaxin in the male. Biol Reprod. 1989 Feb;40(2):197–200. doi: 10.1095/biolreprod40.2.197. [DOI] [PubMed] [Google Scholar]