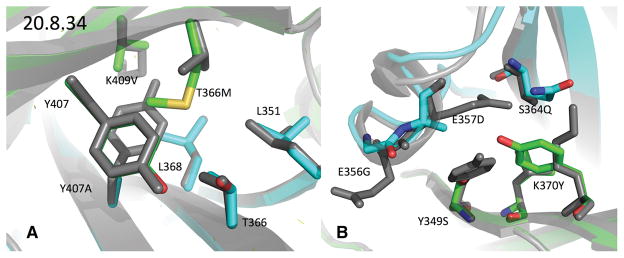
Figure 5. Comparison of design 20.8.34’s crystal structure with WT.
The crystal structure of design 20.8.34 (chain A: green, chain B: cyan) is aligned by its interface residues with the WT crystal structure 1L6X (grey). A) The mutations from 7.4−1 and A_T366M. The rotamer adopted by A_T366M helps explain why Rosetta never predicted this mutation to improve 20.8: at −148°, its χ2 angle is 30° off from the nearest rotamer Rosetta would have sampled. This conformation appears to relieve the collision it would otherwise have had with B_L351. Additionally, the A_T366M’s Cα-Cβ bond vector swings by 4 degrees, and Cα shifts 0.2A, both motions separating the methionine’s sulfur from BL351. Rosetta did however suggest T366M in design 6.1, the crystal structure of which matched the design model closely (Figure S5). B) The mutations from 11.2 and B_E356G. The largest changes in this structure were seen in the small helix from 355 to 358 on the B chain so B_E357D’s side chain ended up unresolved in the structure and seems to no longer contact A_K370Y. The interface-residue, heavy-atom RMSD between the two structures is Å.