Abstract
Background:
Hypertrophic lichen planus (HLP) classically involves shin and ankles and is characterized by hyperkeratotic plaques and nodules. Prurigo nodularis (PN) is a chronic neurodermatitis that presents with intensely pruritic nodules. Histopathology of HLP and PN demonstrate epidermal hyperplasia, hypergranulosis, and compact hyperkeratosis. The dermis shows vertically arranged collagen fibers and an increased number of fibroblasts and capillaries in both conditions. Moreover, basal cell degeneration is confined to the tips of rete ridges, and band-like infiltration is conspicuously absent in HLP. Therefore, both conditions mimic each other clinically, which makes diagnosis difficult. Hence, there is a need for a diagnostic technique to differentiate both conditions.
Objective:
To evaluate dermoscopic patterns in HLP and PN and to study these patterns histopathologically.
Materials and methods:
The study was conducted at S. Nijalingappa Medical College in Bagalkot. It was an observational case series study. Ethical clearance and informed consent was obtained. A Dermlite 3 dermoscope (3Gen, San Juan Capistrano, CA, USA) attached to a Sony Cyber Shot camera DSC-W800 (Sony Electronics Inc., San Diego, California, USA) was employed. Histopathology was done to confirm the diagnosis.
Results:
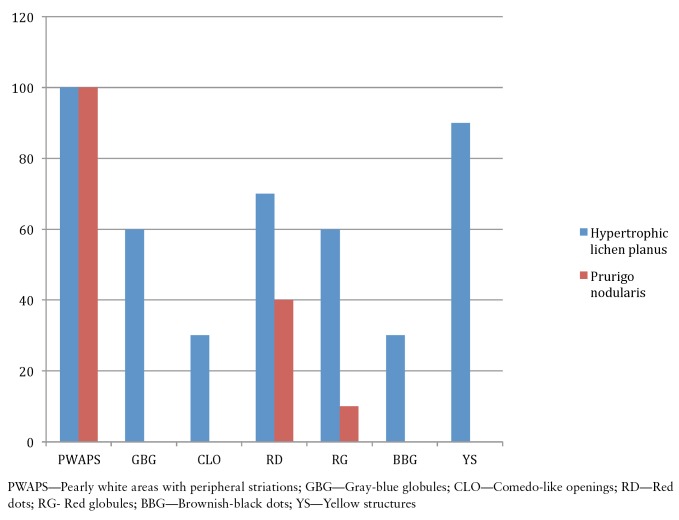
There were 10 patients each with HLP and PN. HLP was seen in 8 males and 2 females. PN was observed in 7 females and 3 males. Dermoscopy of HLP demonstrated pearly white areas and peripheral striations (100%), gray-blue globules (60%), comedo-like openings (30%), red dots (40%), red globules (10%), brownish-black globules (30%), and yellowish structures (90%). In PN, red dots (70%), red globules (60%), and pearly white areas with peripheral striations (100%) were observed under dermoscopy.
Conclusion:
Both HLP and PN demonstrated specific dermoscopic patterns which can be demonstrated on histopathologic findings. The authors propose that these patterns are hallmarks of each condition. Thus, dermoscopy is a good diagnostic tool in the differentiation of HLP and PN.
Keywords: dermoscopy, hypertrophic lichen planus, prurigo nodularis, histopathology, patterns
Introduction
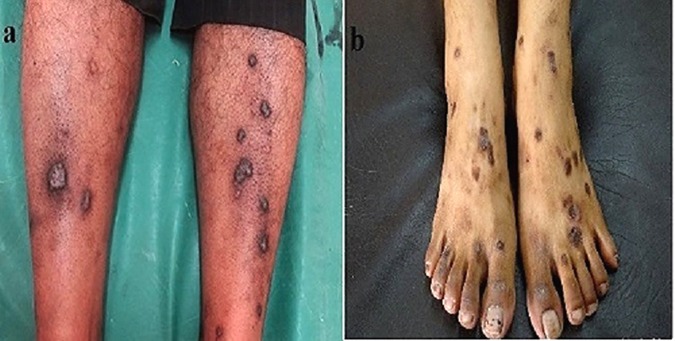
Hypertrophic lichen planus (HLP) is the second most common cutaneous variant of lichen planus. It is characterized as extremely pruritic, and thick hyperkeratotic plaques are seen primarily on the shins or dorsal aspect of the foot and may be covered by a fine adherent scale. The lesions are usually symmetrical and tend to be chronic because of repetitive scratching. Later, lesions become hyperkeratotic thickened elevated purplish or reddish plaques and nodules. The average duration of HLP in patients whose lesions had cleared was reported to be 6 years. Chronic venous stasis frequently contributes to the development of this condition (Figure 1a) [1,2].
Figure 1.
(a) Hyperkeratotic, thickened, plaques and nodules on the legs in hypertrophic lichen planus. (b) Hyperkeratotic papules, plaques and nodules on the legs and dorsum of feet in prurigo nodularis. [Copyright: ©2016 Ankad et al.]
Prurigo nodularis (PN) is a chronic, benign neurodermatitis of unclear etiology characterized by excoriated, intensely pruritic nodules, which are secondary to an intense itch-scratch cycle (Figure 1b). It was first described by Hardaway in 1880 and named by Hyde and Montgomery in 1909 [2]. It is found on exposed extensor surfaces of the lower extremities. Vigorous scratching or rubbing results in lichenification, neurotic excoriations, and nodulation [3,4]. HLP and PN mimic each other clinically, especially when HLP affects the lower legs [3, 5].
Histopathology of HLP reveals epidermal hyperplasia, acanthosis, hypergranulosis and compact and lamellated hyperkeratosis centered on follicular infundibula and acrosyringia. Basal cell damage is usually confined to the tips of rete ridges and may be missed on casual observation [6]. Band-like infiltration is distinctly missing in the dermis [7]. These pitfalls in histopathology of HLP make it difficult to diagnose histopathologically, unlike classical LP. Collagen bundles are oriented vertically in the papillary dermis in association with an increased number of eosinophils [6].
The characteristic histopathology of PN is the presence of thick, compact orthohyperkeratosis, irregular epidermal hyperplasia or pseudoepitheliomatous hyperplasia, focal parakeratosis, and hypergranulosis. The papillary dermis shows fibrosis with vertically arranged collagen fibers and increased number of fibroblasts and capillaries [8]. Hence, histopathologic differentiation of both HLP and PN is difficult in a few instances. The authors evaluated the dermoscopic patterns in both conditions and believe that these patterns were specific to each condition that would help in differentiating two diseases.
Materials and methods
The study was conducted in the Department of Dermatology in a tertiary hospital attached to S. Nijalingappa Medical College at Bagalkot in Southern India from October 2014 to July 2015. It was an observational case series study. Ten patients each with clinical signs and symptoms of HLP and PN were subjected to complete history and dermatological examination. Ethical clearance was obtained by the institutional ethical committee. Informed written consent was taken from patients. Demographic data such as age, gender and disease duration were all documented.
Dermoscopic examination
A DermLite 3 dermoscope (with 10× magnification) with both polarized and non-polarized lights was employed in the study. Sony digital camera (14 Megapixels) was attached to save the images. Initially, ultrasound gel was applied either on the faceplate of the dermoscope or on the skin lesions and then lesions were observed through the eyepiece of the dermoscope. However, only the polarized light version was used in our study to appreciate color patterns in the dermis. Although polarized dermoscopy was employed, ultrasound gel was applied for clarity of images and to lessen distortions associated with light [9].
All new and old lesions of HLP and both excoriated and hyperkeratotic lesions of PN were examined under dermoscopy. Data was tabulated in a Microsoft Excel® sheet. Proportions and percentages were used for representing the data. Histopathology was carried out in both HLP and PN to confirm the diagnosis by taking a punch biopsy from each type of lesion.
Inclusion criteria:
Patients with signs and symptoms of HLP and PN.
Patients who had not received or stopped treatment for HLP and PN 1 month prior to the study.
Exclusion criteria:
Patients with secondary infection superseding HLP and PN.
Patients who were receiving treatment 1 month prior to the study.
Results
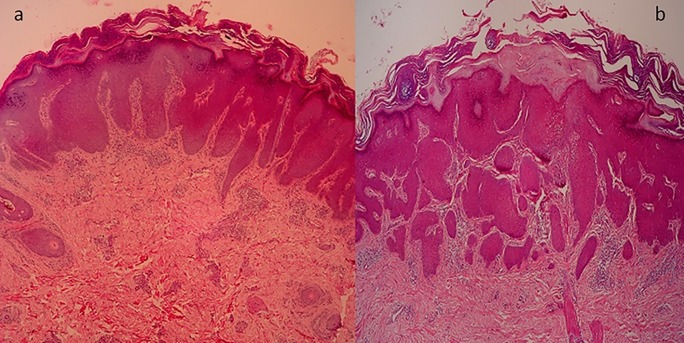
Out of 10 HLP, 8 male and 2 female patients were present between the ages of 26 and 50 years (mean age 38 years). Duration of disease was between 4 months and 48 months. Dermoscopy demonstrated milky white structures at the center and grayish strands which were arranged peripherally. This was referred to as pearly white areas (Wickham’s striae) and peripheral striations (Figures 2–4). Gray-blue globules were present diffusely in the center extending peripherally (Figure 3). Comedo-like openings (CLO) were observed as regular dells filled with keratin on the surface and were situated diffusely over the lesions (Figures 3, 4). Yellowish structures observed were arranged in a lacy network over the lesions (Figure 4). Tiny pigmented areas referred to as brownish-black globules were present at the periphery of the lesions (Figure 3). Red dots and red globules were present at the center and periphery (Figures 3, 4). Histopathology of HLP demonstrated orthokeratosis, hypergranulosis, and elongation of rete ridges (Figure 5a). Dermoscopic patterns with corresponding histopathologic changes are tabulated in Table 1.
Figure 2.
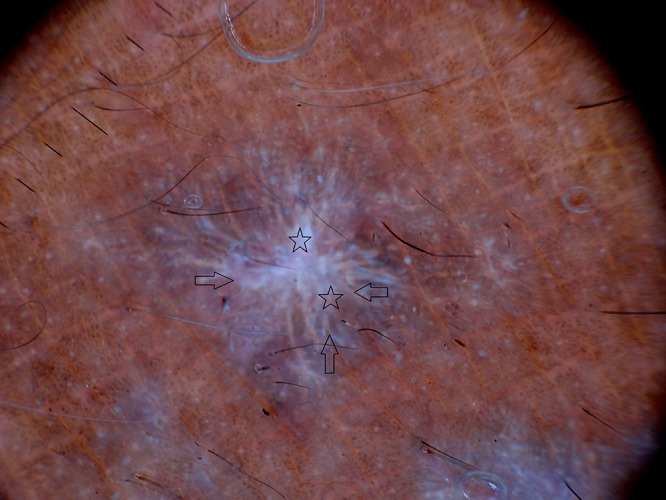
Dermoscopy of hypertrophic lichen planus shows pearly white areas (stars), peripheral striations (arrows). [Copyright: ©2016 Ankad et al.]
Figure 3.
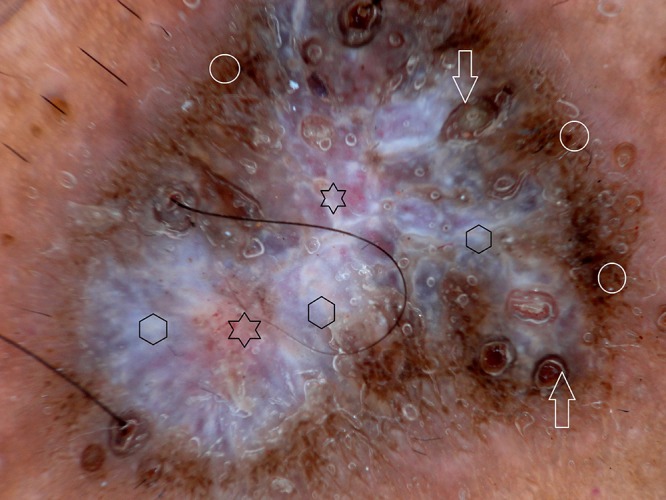
Dermoscopy of hypertrophic lichen planus shows gray-blue globules (hexagons), comedo-like openings (arrows), brownish-black dots (circles) and red globules (stars). [Copyright: ©2016 Ankad et al.]
Figure 4.
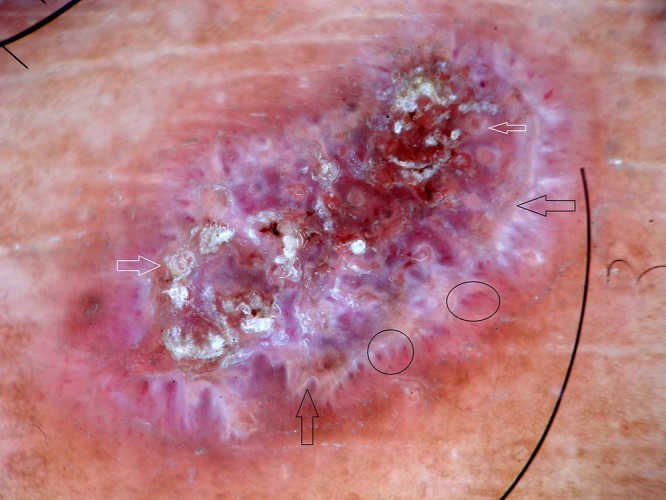
Dermoscopy of hypertrophic lichen planus shows yellowish structure (black arrows), comedo-like openings (white arrows) and peripheral blood vessels (circles). [Copyright: ©2016 Ankad et al.]
Figure 5.
(a) Histopathology of hypertrophic lichen planus with compact orthokeratosis, hypergranulosis and elongation of rete ridges. (b) Histopathology of prurigo nodularis with orthokeratosis and hyperkeratosis; irregular acanthosis and elongated rete ridges. (hematoxylin & eosin, ×4). [Copyright: ©2016 Ankad et al.]
TABLE 1.
Proposed dermoscopic patterns corresponding to histopathologic features in hypertrophic lichen planus. [Copyright: ©2016 Ankad et al.]
| Dermoscopic patterns | Corresponding histopathologic changes | |
|---|---|---|
| 1 | Pearly white areas (Wickham striae); and peripheral striations | Compact orthokeratosis above zones of wedge-shaped hypergranulosis, acanthosis, and dermal fibrosis. |
| 2 | Gray-blue globules | Dermal melanophages |
| 3 | Comedo-like openings | Hypergranulosis and hyperkeratosis of dilated infundibulum |
| 4 | Red dots | Dermal capillaries |
| 5 | Red globules | Dermal capillaries |
| 6 | Brownish-black globules | Epidermal melanocytes |
| 7 | Yellow structures | Spongiosis and vacuolar degeneration of basal cell |
PN was observed in 7 female and 3 male patients with ages ranging from 20 to 54 years. Mean duration of disease was 4 years (minimum 1 year and maximum 7 years). Histopathology of PN showed orthokeratosis and hyperkeratosis, irregular acanthosis and elongated rete ridges (Figure 5b). Dermoscopy of PN demonstrated pearly white structures in the center with peripheral extensions. The pattern is described as “starburst” appearance. These white areas were surrounded by peripheral striations (Figure 6). Red dots and red globules (Figures 6, 7) were located diffusely in the center. Dermoscopic patterns and corresponding histopathologic changes are represented in Table 2. Frequencies of each dermoscopic pattern in HLP and PN are shown in Figure 8.
Figure 6.
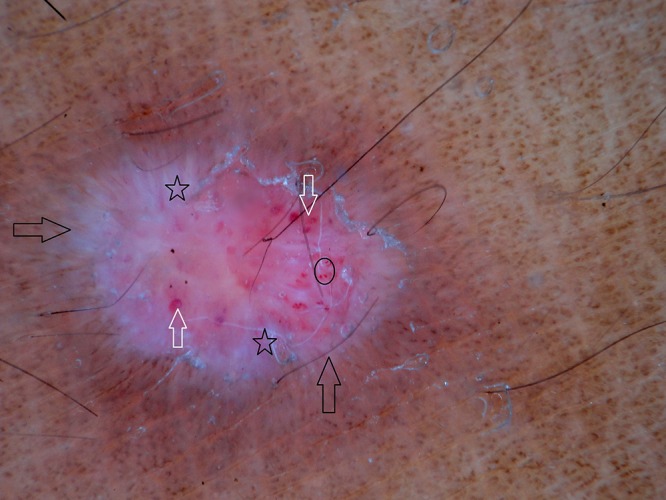
Dermoscopy of prurigo nodularis shows pearly white areas (stars), red globules (circle), red areas (white arrows) and peripheral striations (black arrows). [Copyright: ©2016 Ankad et al.]
Figure 7.
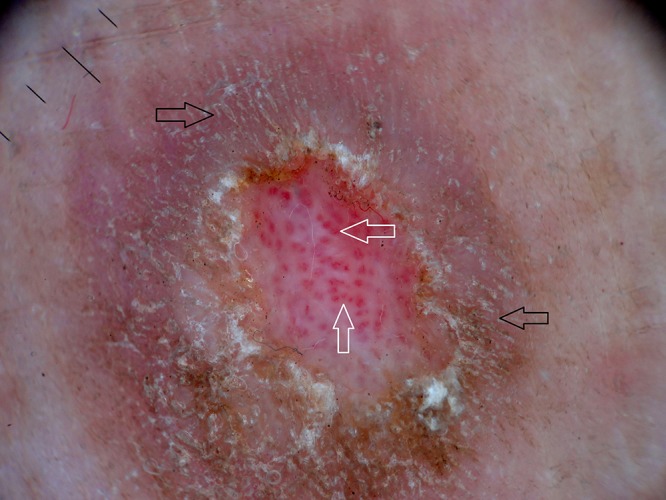
Dermoscopy of prurigo nodularis shows red globules (white arrows) and peripheral striations (black arrows). [Copyright: ©2016 Ankad et al.]
TABLE 2.
Proposed dermoscopic patterns corresponding to histopathologic features in prurigo nodularis. [Copyright: ©2016 Ankad et al.]
| Dermoscopic patterns | Corresponding histopathologic changes | |
|---|---|---|
| 1 | White areas and peripheral striations | Hyperkeratosis, hypergranulosis, acanthosis and dermal fibrosis |
| 2 | Red dots | Dilated capillaries |
| 3 | Red globules | Focal hemorrhages |
Figure 8.
Frequency of dermoscopic patterns in hypertrophic lichen planus and in prurigo nodularis. [Copyright: ©2016 Ankad et al.]
Discussion
HLP develops during the course of a subacute attack, nevertheless, occasionally only hypertrophic or warty lesions are found. The most common site is the lower limbs, especially around the ankles. The development of hypertrophic lesions greatly lengthens the course of the disease, as they may persist for many years. HLP must be distinguished from lichen simplex chronicus, PN and lichen, amyloidosis [10].
Dermoscopy of HLP and PN showed pearly white areas, which were more prominent in PN than in HLP. There was a slight difference in the appearance. White areas were spread over the whole periphery of the lesion in PN and it was localized in the center with peripheral extensions in some areas in HLP. Peripheral extensions were well defined in PN giving a “starburst” appearance.
In classic lichen planus, pearly white areas are arranged in annular and arboriform pattern or circular, linear, globular, reticular and radial streaming [2,11]. However, there was no mention of HLP in these studies. Different configurations and arrangements of pearly white areas help to differentiate HLP, PN, and classical lichen planus. Pearly white areas correspond histopathologically to compact orthokeratosis above zones of wedge-shaped hypergranulosis and acanthosis [12]. Peripheral striations were more pronounced in PN than in HLP, and this pattern corresponds to dermal fibrosis in histopathology [13].
Other conditions which can be listed in the differential diagnosis of HLP and PN by dermoscopy include nodular scabies, keratoacanthoma, and reactive perforating collagenosis [14].
CLO filled with yellow keratinous plugs referred to as corn pearls were observed in HLP by Vazquez-Lopez F et al [12]. CLO correspond to dilatation, plugging and hypergranulosis of infundibulum and they are suggestive of transepithelial elimination. In HLP, histopathologic changes, namely, epidermal hyperplasia, acanthosis, hypergranulosis and compact and lamellated hyperkeratosis, are centered on follicular infundibula and acrosyringia [15]. Hence, CLO are very specific to hypertrophic LP and are not observed in PN. CLO are also demonstrated in the early stages of lichen sclerosis and in basal cell carcinoma and seborrheic keratosis [16–18].
Gray-blue globules observed in this study represent melanin pigment in the dermis due to melanin incontinence as a result of vacuolar degeneration. The configuration of gray-blue globules and dots is specific for each condition. Gray-blue globules in lichen planus pigmentosus of the scalp appear as a “target” pattern. This suggests that pathology is around the perifollicular area and spares the interfollicular area. In discoid lupus erythematosus of the scalp, the pathologic process involves perifollicular and interfollicular areas, hence, gray-blue globules follow a “speckled” pattern [19]. Gray-blue globules appear as ovoid and nest-like in basal cell carcinoma [17]. In this study, gray-blue globules in HLP were arranged in diffuse structureless pattern interspersed in pearly white areas indicating presence of melanin incontinence in perifollicular and interfollicular areas. Gray-blue globules were not demonstrated in PN, suggesting absence of melanin incontinence in PN.
Yellow structures represent vacuolar degeneration of the basal layer and spongiosis [2]. Yellow structures demonstrated in this study appear as a “lacy network” pattern, traversing the pearly white areas. The characteristic situation of CLO along yellow structures confirms the fact that the pathological process in HLP is cornered in and around follicles. Yellow structures were not demonstrated in PN. Nevertheless, brown-yellowish crusts were noted in PN in one study [13].
Melanocytes in the epidermis appear as brownish-black globules in dermoscopy and their arrangement is diffuse, annular or in dotted patterns in classical LP [2]. In HLP, brownish-black globules were diffusely arranged surrounding gray-blue dots. Brownish-black dots were not observed in PN.
Red dots correspond to dilated capillaries in histopathology [20]. Arrangement and configuration of red globules give a clue to the condition. Red dots were seen as red indistinct islands in the confines of pearly white structures in this study. They were observed in both HLP and PN. They were centrally located in PN in a “comma-like” pattern, whereas in HLP they were arranged diffusely in the lesions. In psoriasis, they appear as regular dotted vessels over a light red background, and in pityriasis rosea, arrangement of vessels is patchy on a yellow background. In classic LP, vessels are arranged peripherally in the confines of white crossing lines and they appear as clear red globules [20].
Red globules are larger than the red dots. These were prominent in PN and were not evidently seen in HLP in this study. These correspond to enlarged blood vessels as well as focal hemorrhage in dermis. Similar findings, in addition to brown-yellowish crusts and scales, were observed by Errichetti et al in excoriated and hyperkeratotic lesions of PN [13]. However, yellow areas and crusts were not demonstrated in this study. Description of dermoscopy of HLP with histopathologic correlation is well documented [21] and similar dermoscopic patterns were observed in the present study.
Histopathologic diagnosis of a condition depends on the characteristic features of that condition which may not be observed in all lesions submitted for histopathologic examination [22]. Dermoscopy visualizes the color patterns in the epidermis, dermo–epidermal junction, and papillary dermis; when these patterns are observed consistently in a given disease, they could aid in its diagnosis [23]. Although the same dermoscopic patterns are expected in HLP and PN due to a few similar histopathologic findings, dermoscopy demonstrated some different patterns which are specific in each condition. This is probably because of the few specific histopathologic features which are unique to HLP and PN.
Conclusion
Dermoscopy is an in vivo diagnostic technique enabling clinicians to visualize subsurface structures with appropriate configuration and color patterns. HLP and PN demonstrate specific dermoscopic patterns that correspond to histopathologic findings. Gray-blue globules, CLO and brownish-black globules were specific to HLP. Hence, the authors propose that these patterns are a hallmark of each condition. Thus, dermoscopy is helpful in the differentiation of HLP and PN. Further studies involving large sample size are suggested for studying the validity (sensitivity, specificity) of dermoscopy in making the diagnosis.
Acknowledgments
The authors wish to acknowledge the help of Dr. Vijay Domble for his assistance with histopathology.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. New York: Elsevier Saunders; 2012. pp. 183–202. [Google Scholar]
- 2.Doshi B, Khopkar U. Histopathology of lichen planus and its variants. In: Khopkar U, Valia A, editors. Lichen Planus. New Delhi: Jaypee Brothers Medical Publisher (P) LTD; 2013. pp. 123–47. [Google Scholar]
- 3.Vaidya DC, Robert A. Schwartz. Prurigo nodularis: a benign dermatosis derived from a persistent pruritus. Acta Dermatovenerol Croat. 2008;16:38–44. [PubMed] [Google Scholar]
- 4.Lee MR, Shumack S. Prurigo nodularis: A review. Aust J Dermatol. 2005;46:211–20. doi: 10.1111/j.1440-0960.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 5.Berth-Jones J. Eczema, Lichenification, prurigo and erythroderma. In: Burns T, Breathnach SM, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 8th ed. 23. Oxford: Wiley-Blackwell; 2010. pp. 1–51. [Google Scholar]
- 6.Weedon D. The lichenoid reaction pattern (interface dermatitis) In: Weedon D, editor. Weedon’s Skin Pathology. 2nd ed. London: Churchill Livingstone; 2002. pp. 31–74. [Google Scholar]
- 7.Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular and squamous diseases. In: Elder DE, editor. Lever’s Histopathology of the Skin. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. pp. 169–204. [Google Scholar]
- 8.Weigelt N, Metze D, Ständer S. Prurigo nodularis: Systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578–86. doi: 10.1111/j.1600-0560.2009.01484.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowling J. Introduction to dermoscopy. In: Bowling J, editor. Diagnostic Dermoscopy: The Illustrated Guide. West Sussex: Wiley-Blackwell; 2012. pp. 2–14. [Google Scholar]
- 10.Breathnach SM. Lichen planus and lichenoid disorders. In: Burns T, Breathnach SM, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 8th ed. 41. Oxford: Wiley-Blackwell; 2010. pp. 1–28. [Google Scholar]
- 11.Gungor S, Topal IO, Goncu EK. Dermoscopic patterns in active and regressive lichen planus and lichen planus variants: a morphological study. Dermatol Pract Concept. 2015;5(2):45–53. doi: 10.5826/dpc.0502a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez-Lopez F, Manjon-Haces JA, Maldonado-Seral C, et al. Dermoscopic features of plaque psoriasis and lichen planus: new observations. Dermatology. 2003;207:151–6. doi: 10.1159/000071785. [DOI] [PubMed] [Google Scholar]
- 13.Errichetti E, Piccirillo A, Stinco G. Dermoscopy of prurigo nodularis. J Dermatol. 2015;42:632–34. doi: 10.1111/1346-8138.12844. [DOI] [PubMed] [Google Scholar]
- 14.Errichetti E, Stinco G. The practical usefulness of dermoscopy in general dermatology. G Ital Dermatol Venereol. 2015;150(5):533–46. [PubMed] [Google Scholar]
- 15.Busam KJ, Goldblum JR, editors. Dermatopathology: A Volume in a Series: Foundations in Diagnostic Pathology. Philadelphia: Saunders Elsevier; 2010. pp. 11–81. [Google Scholar]
- 16.Shim WH, Jwa SW, Song M, et al. Diagnostic usefulness of dermatoscopy in differentiating lichen sclerosus et atrophicus from morphea. J Am Acad Dermatol. 2012;66:690–1. doi: 10.1016/j.jaad.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Bowling J. Non-melanocytic lesions. In: Bowling J, editor. Diagnostic Dermoscopy: The Illustrated Guide. West Sussex: Wiley-Blackwell; 2012. pp. 59–91. [Google Scholar]
- 18.Braun RP, Rabinovitz HS, Krischer J, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol. 2002;138:1556–60. doi: 10.1001/archderm.138.12.1556. [DOI] [PubMed] [Google Scholar]
- 19.Ankad BS, Beergouder SL, Moodalgiri VM. Lichen planopilaris versus discoid lupus erythematosus: A trichoscopic perspective. Int J Trichol. 2013;5:204–7. doi: 10.4103/0974-7753.130409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br Journal Dermatol. 2012;166:1198–205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 21.Ankad BS, Beergouder SL, Sujana L. Dermoscopy of hypertrophic lichen planus. Austin J Dermatolog. 2014;1(3):1013. http://austinpublishinggroup.com/dermatology/fulltext/ajd-v1-id1013.php. Accessed on August, 20, 2015. [Google Scholar]
- 22.Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papules and squamous diseases. In: Elder DE, editor. Lever’s Histopathology of the Skin. 10th ed. Philadelphia: Lippincott-Williams Wilkins; 2009. pp. 169–203. [Google Scholar]
- 23.Nischal KC, Khopkar U. Dermoscope. Indian J Dermatol Venereol Leprol. 2005;71:300–3. doi: 10.4103/0378-6323.16633. [DOI] [PubMed] [Google Scholar]