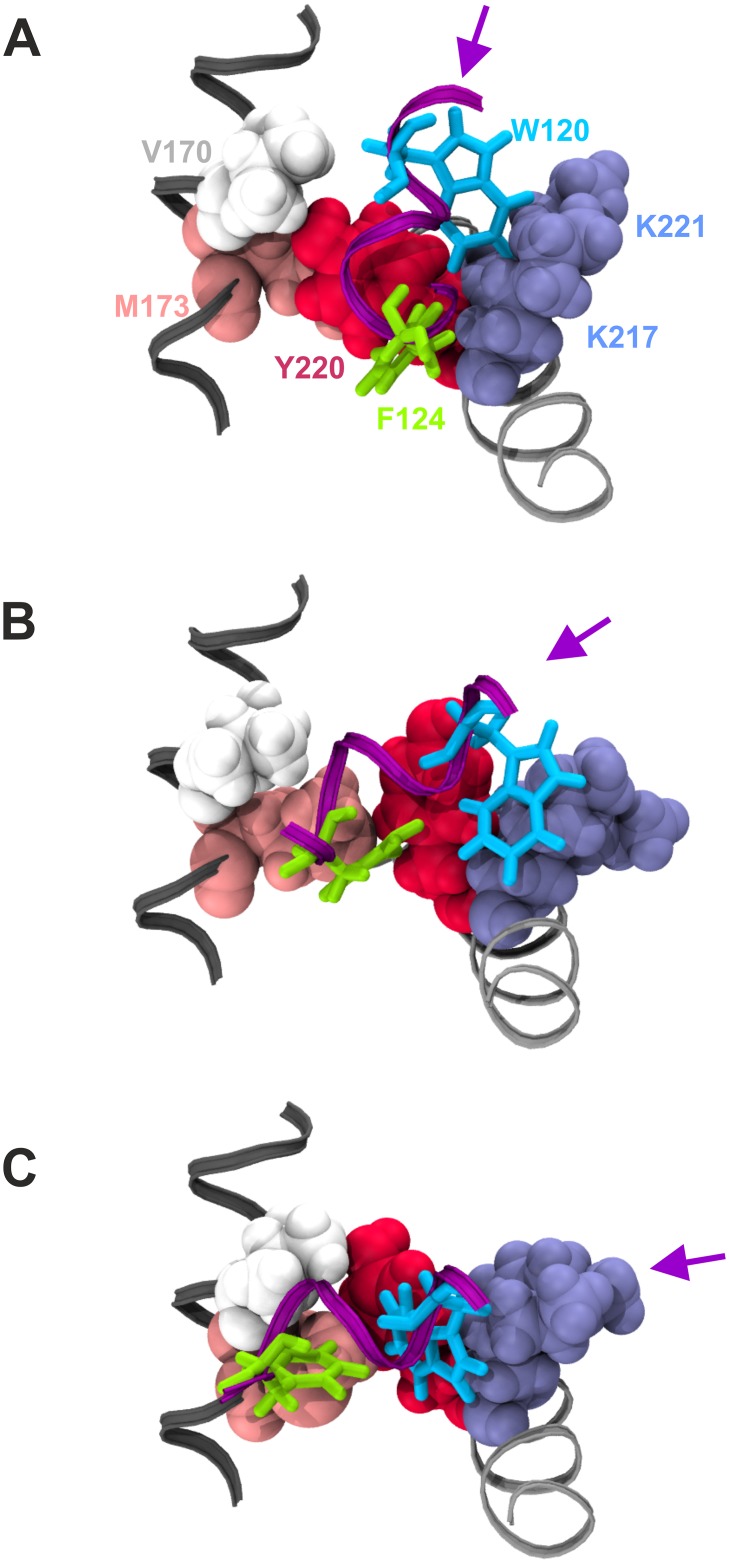
Fig 7. Structural basis of pocket #1 occupancy by GCN4-cAD residues W120 and F124.
GCN4-cAD residues W120 and F124 are displayed in liquorice representation in light blue and green, respectively, on a portion of the GCN4 α-helix shown as a purple ribbon. Key residues participating in the formation of Pocket #1 on the GAL11-ABD1 surface are drawn as van der Waals structures and labelled according to amino acid identiy (V170 white; M173 pink; Y220 red; K217 and K 221 in light blue). V170 and M173 are part of GAL11-ABD1 α-helix 1 (see Fig 2B for helix nomenclature) marked in dark grey. K217, K221 and Y220 are on GAL11-ABD1 α-helix 4 shown in light grey. The changing orientation of the GCN4 α-helical axis is marked by a purple arrow. A. Structure near beginning of simulation (30 ns of GAL11-ABD1/GCN4-cAD _aMD_no1). GCN4-F124 occupies Pocket #1 formed by GAL11-Y220 and -K217 (among other residues not shown here), while GCN4-W120 engages in hydrophobic interactions with the non-polar section of the GAL11-K221 side-chain. B. Structure after 120 ns simulation (GAL11-ABD1/GCN4-cAD _aMD_no1). GCN4-F124 has moved towards GAL11-M173, freeing up Pocket #1 for GCN4-W120 to move in while maintaining hydrophobic engagements with GAL11-K217 and -K221. C. Structure after 220 ns simulation (GAL11-ABD1/GCN4-cAD _aMD_no1). GCN4-F124 binds now to GAL11-M173 and -V170, and Pocket #1 is now fully occupied by GCN4-W120.