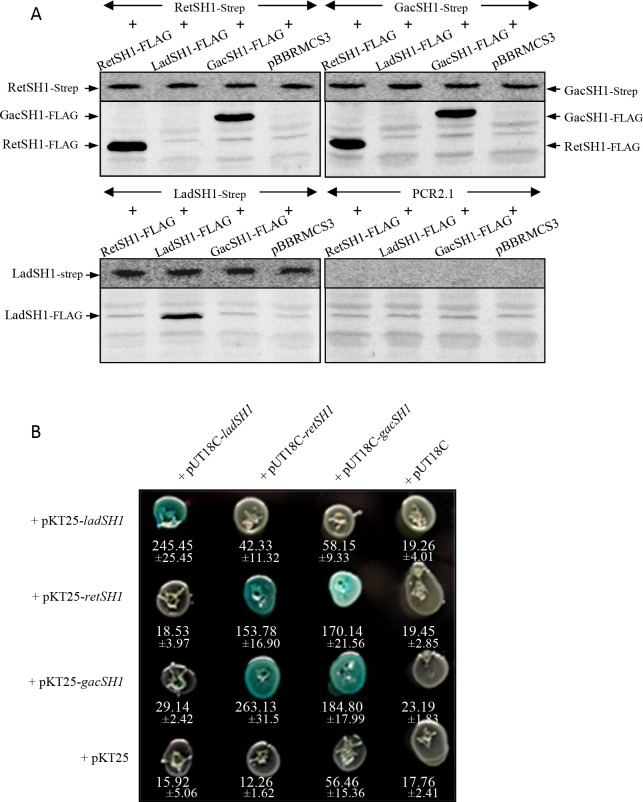
Fig 4. Interactions between H1 domains of the LadS hybrid HK, the GacS unorthodox HK and the RetS hybrid HK using pull-down and two-hybrid experiments.
(A) Pull-down experiments. N-terminal FLAG or Strep versions of the H1 domain of GacS, LadS and RetS HKs were constructed in pBBRMCS3 and pCR2.1 vectors, respectively, and expressed in E. coli. Cell lysates were immunoprecipitated using anti-Strep antibody-coupled beads, and FLAG and Strep derivatives were further detected using StrepTactin Alkaline Phosphatase conjugate (upper panel) and anti-FLAG antibody detection (lower panel). (B) In two-hybrid experiments, the ladSH1, retSH1 and gacSH1 DNA regions were cloned into the two-hybrid pUT18C or pKT25 vectors and corresponding vectors were co-transformed in BTH101 cells that were further streaked on LB plates containing X-gal. A blue color of colonies reflects interaction between chimeric proteins, while white color attests to the absence of interaction. The interactions were further quantified by measuring the corresponding ß-galactosidase levels expressed in Miller units (values and standard deviations of 3 independent clones below corresponding colonies).