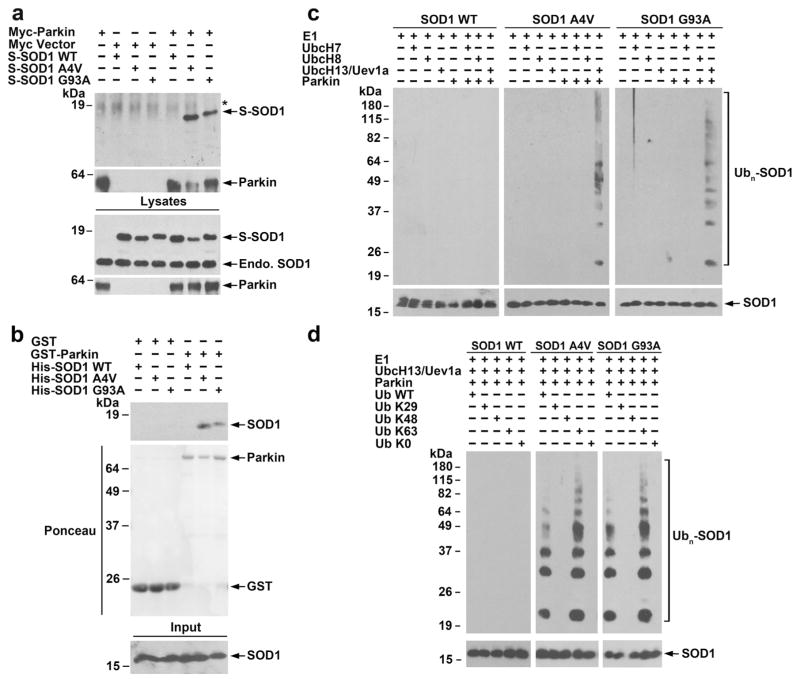
Fig. 2.
Selective interaction and ubiquitination of mutant SOD1 by parkin. a Co-immunoprecipitation analysis reveals a specific interaction of parkin with SOD1 A4Vand G93A mutants, but not SOD1 WT. Lysates from transfected SH-SY5Y cells were subjected to immunoprecipitation with anti-Myc antibody followed by immunoblotting with anti-Myc antibody and anti-SOD1 and antibody, which recognized both S-tagged and endogenous (endo.) SOD1 proteins. Asterisk denotes nonspecific band. b In vitro binding assays were performed by incubation of soluble His-tagged SOD1 WT or mutants (input) with immobilized GST or GST-parkin (shown by Ponceau stain). Analysis of bound proteins by immunoblotting with anti-SOD1 antibody shows direct binding of mutant SOD1 to parkin. c In vitro ubiquitination analysis of purified SOD1 WT or mutants in the presence of indicated E1, E2 (UbcH7, UbcH8, or UbcH13/Uev1a), GST-parkin or GST, and ubiquitin reveals selective ubiquitination of mutant SOD1 by parkin in cooperation with the UbcH13/Uev1a E2 enzyme. d In vitro ubiquitination analysis of SOD1 WT or mutants in the presence of E1, UbcH13/Uev1a, GST-parkin, and indicated wild-type or mutant ubiquitin shows that in vitro polyubiquitination of mutant SOD1 by parkin occurs via the K63-linkage