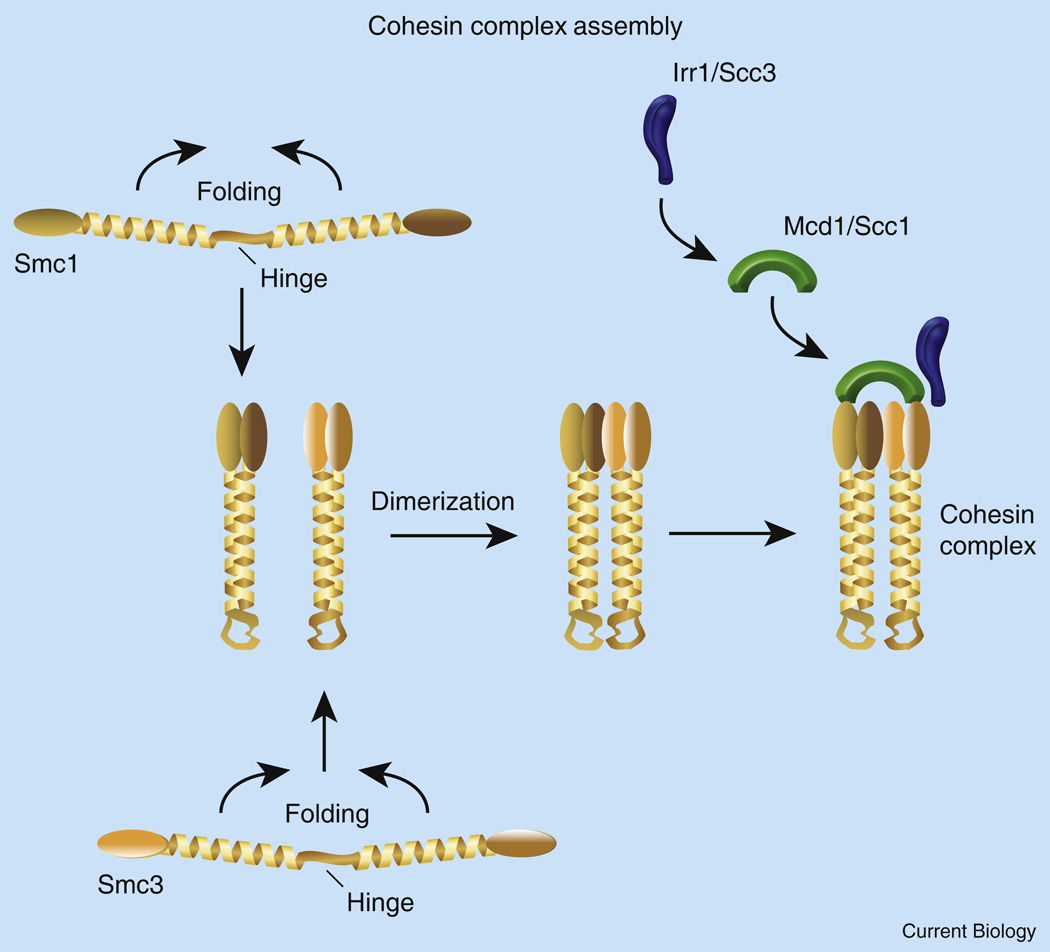
Figure 1. Cohesin complex assembly and structure.
SMCs contain long helical domains interrupted by a centrally positioned hinge. Hinge folding enables the helical domains to intertwine in an anti-parallel fashion and form a stable coiled-coil rod-like structure. Folding over also brings amino- and carboxy-terminal globular ATPase domains for each SMC into registration. Smc1/3 bind together by dimerization of hinge domains and by association of globular head domains. The Smc1/3 ATPase domains are capped by Mcd1/Scc1. Mcd1/Scc1 recruits Scc3/Irr1.