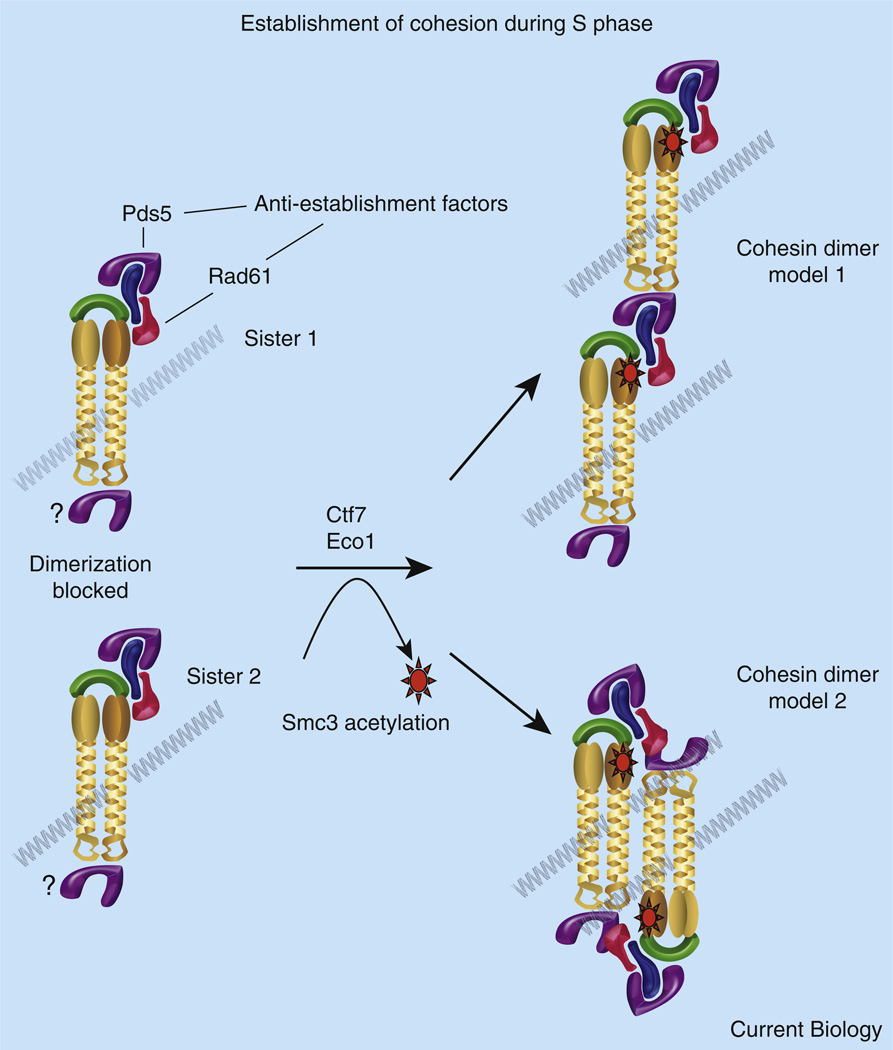
Figure 3. Cohesion establishment.
Shown is a speculative model regarding the conversion of cohesins to a cohesion-competent state during S phase. Cohesin complexes deposited onto (or entrapping) each chromatid are separate and distinct entities. Recruitment of anti-establishment factors Pds5 and WAPL/Rad61 onto Scc3/Irr1 precludes the tethering together of cohesin complexes by destabilizing cohesin–cohesin association. Lines of evidence equally support recruitment of Pds5 to both Scc3/Irr1 and to hinge dimerization domains, suggesting that either cohesin terminus may participate in pairing. Pds5 and WAPL/Rad61 anti-establishment factors may also promote chromatid capture reactions by destabilizing subunit interactions within each cohesin (not shown). During S phase, Ctf7/Eco1 acetylates Smc3 near the ATPase globular domain. Smc3 acetylation temporarily inhibits anti-establishment activity, possibly by altering the association or binding site of WAPL/Rad61 and Pds5 to the rest of the cohesin complex (note rotation of anti-establishment factors shown for instance in model 2). Dimerization of cohesins associated with each sister chromatid results in a stable structure that is resistant to antiestablishment activity and persistent Smc3 acetylation. Two of several oligomeric structures (model 1 and model 2) that account for both DNA entrapment and sister chromatid pairing are shown (see [27,28] for a single ring model). In a G2/M response to DNA damage, chromatin-bound cohesins are instead converted to a pairing state by Mcd1 phosphorylation and subsequent acetylation (not shown).