Figure 2.
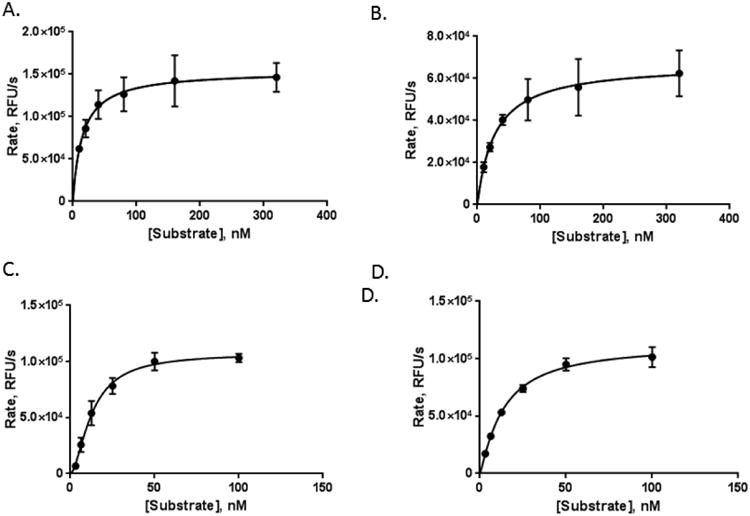
Nicorandil stimulates both wild-type and R177A APE1 endonuclease activity. APE1 endonuclease activity in the absence of nicorandil was measured for wild-type and R177A APE1. Enzyme concentrations of 0.2 nM and 0.0125 nM were used for wild-type and R177A APE1, respectively, for substrate concentrations of 10, 20, 40, 80, 160, and 320 nM. Similarly, the effect of 12.5 μM nicorandil on the kinetics of wild-type (C) and R177A (D) APE1 endonuclease activity was measured. Enzyme concentrations of 0.1 nM for wild-type and 0.0125 nM for R177A APE1 were used with substrate concentrations of 3.13, 6.25, 12.5, 25, 50, and 100 nM. The endonuclease activity was determined by monitoring the release of the fluorescein-labeled product in relative fluorescence units (RFUs) per second over the first five minutes of the reaction and plotted versus the substrate concentration. Curve fitting and kinetic parameters were calculated using GraphPad Prism. Data were obtained from three independent experiments with triplicate measurements for each substrate concentration.
