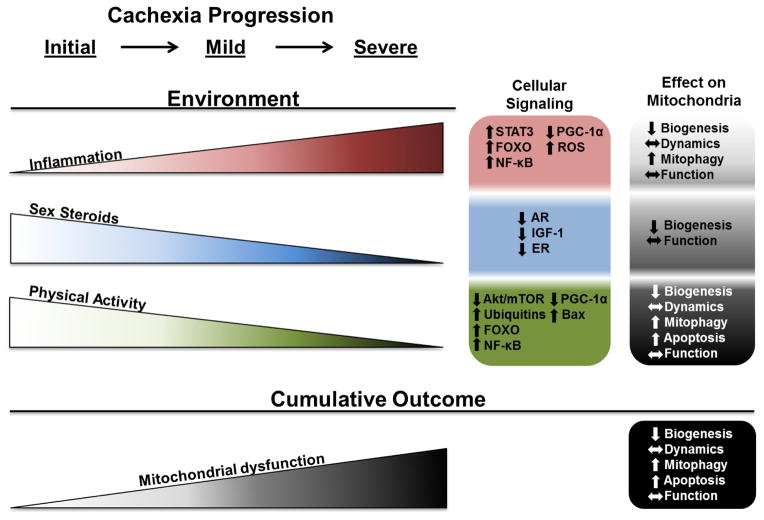
Figure 3. Cancer-induced cachectic environments and their relationship to skeletal muscle mitochondrial dysfunction during the progression of cancer cachexia.
Increases in inflammation and decreases in sex steroids and physical activity disrupt mitochondrial quality throughout the progression of cachexia. Inflammation through systemic interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), TNF-like weak inducer of apoptosis (TWEAK), and myostatin increase throughout cachexia progression result in the activation of STAT3, NF-κB, FOXO, and PGC-1α signaling as well as the generation of reactive oxygen species (ROS). These signaling pathways result in decreased biogenesis, altered dynamics, increased mitophagy, and altered function in cachectic muscle mitochondria. Sex steroids (testosterone and estrogen) and their respective nuclear receptors (androgen and estrogen receptors) decrease throughout cachexia progression. These can negatively regulate anabolic signaling related to insulin-like growth factor 1 (IGF-1). These signaling pathways result in decreased mitochondrial biogenesis and altered mitochondrial function. Physical activity decreases throughout cancer cachexia progression resulting in decreased signaling through Akt/mTOR, and PGC-1α, while increasing signaling through FOXO, NF-κB, Bax, and ubiquitins. Decreased physical activity results in decreased biogenesis, increased mitophagy and apoptosis, and altered mitochondrial dynamics and function. While these factors can work independently, the culmination of systemic factors and decreased use can negatively impact muscle oxidative capacity through the regulation mitochondrial biogenesis, dynamics, mitophagy, apoptosis and function.