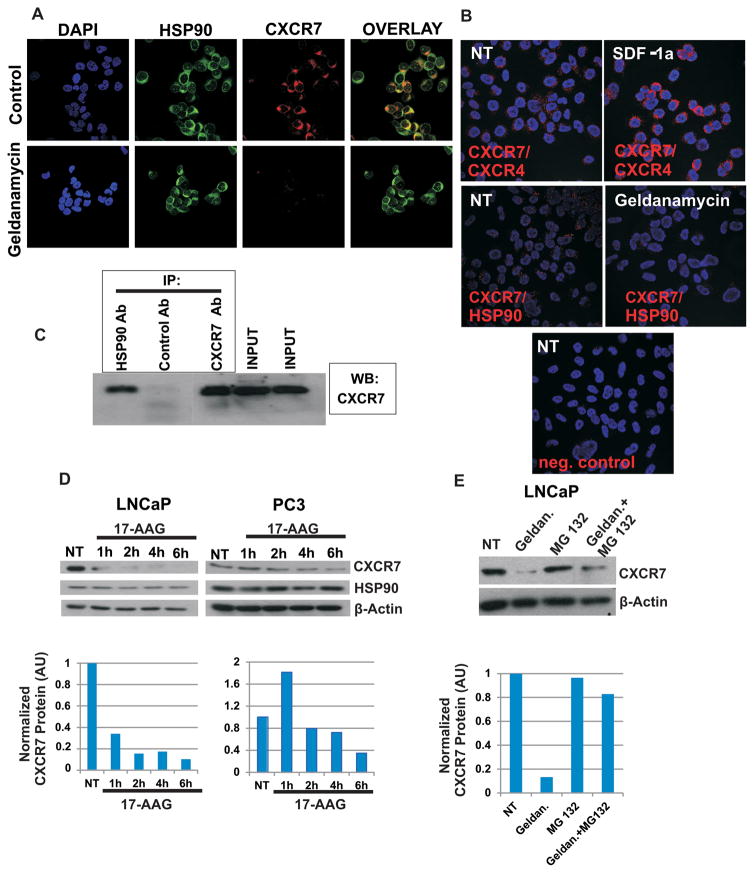
Figure 5. Hsp90 interacts with CXCR7/EGFR/β-Arrestin-2 axis and protects it from proteasome mediated degradation.
A: PC-3 cells were seeded in 8 well chamber slides (20,000 cells per well). Forty-eight hours later, cells were treated with Hsp90 inhibitor Geldanamycin (3 μM, 2 hr) or were left untreated. Cells were fixed with 3% paraformaldehyde, permeabilized with 0.25% Triton X-100 and subjected to double immunofluorescence staining. Expression of Hsp90 was detected by mouse anti-Hsp90 antibody (Enzo Life Sciences, ADI-SPA-830) and Alexa 488 conjugated goat anti-mouse IgG (green). Expression of CXCR7 was analyzed using rabbit anti-CXCR7 antibody (Genetex, GTX100027) and visualized by Alexa 555 conjugated goat anti-rabbit IgG (red). B: PC-3 cells were seeded in 8 well chamber slides (20,000 cells per well). Seventy-two hours later cells were treated with Hsp90 inhibitor Geldanamycin (3 μM, 2 hr) or were left untreated. Cells were fixed with 3% paraformaldehyde and proximity ligation assay (DUOLINK) was performed to detect CXCR7/Hsp90 and CXCR7/CXCR4 co-localization. Mouse anti-Hsp90 antibody (Enzo Life Sciences, ADI-SPA-830) and rabbit anti-CXCR7 antibody (Genetex, GTX100027) were used for detection of Hsp90/CXCR7 co-localization. NT= non-treated cells. C: Co-Immunoprecipitation of CXCR7 and Hsp90: Cell lysates of LNCaP were immunoprecipitated with an anti-Hsp90 IgG followed by western blotting against CXCR7. D: Prostate cancer cells were seeded in 6 well plates. Twenty-four hours later cells were treated with 17-AAG (2 μM) for 1–6 hr, or were left untreated (NT). Protein extracts were collected and subjected to western blotting. Actin served as a loading control. E: LNCaP cells were seeded in 6 well plates. Twenty-four hours later, cells were treated with Geldanamycin (3 μM, 2 hr), proteasome inhibitor MG132 (10 μM, 2 hr) or were left untreated (NT). Protein extracts were subjected to western blotting. Actin served as a loading control. AU: Arbitrary Units