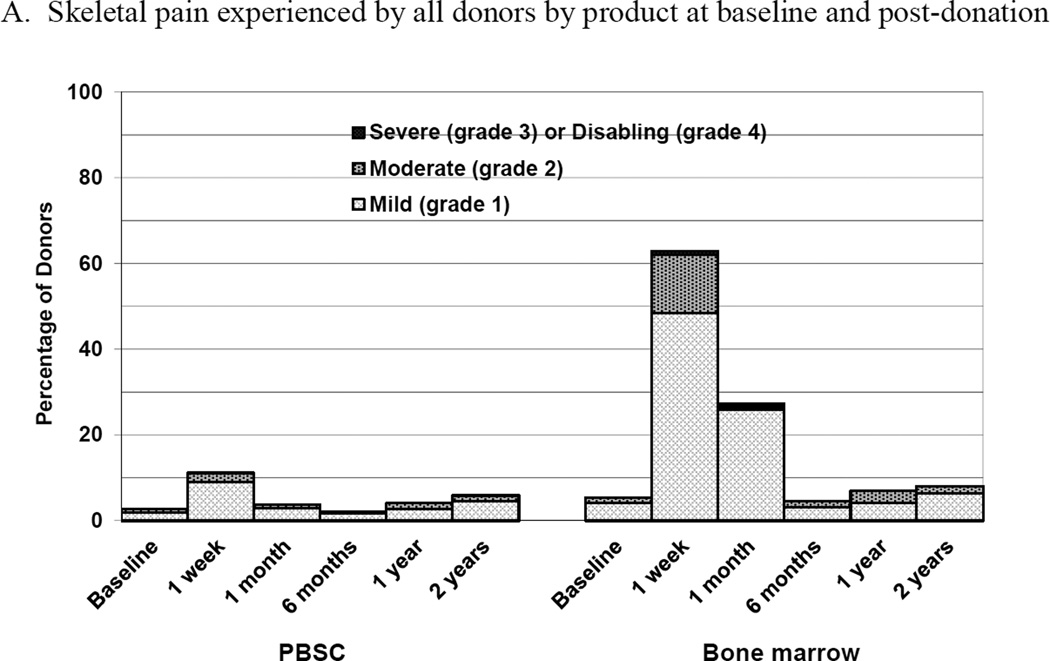
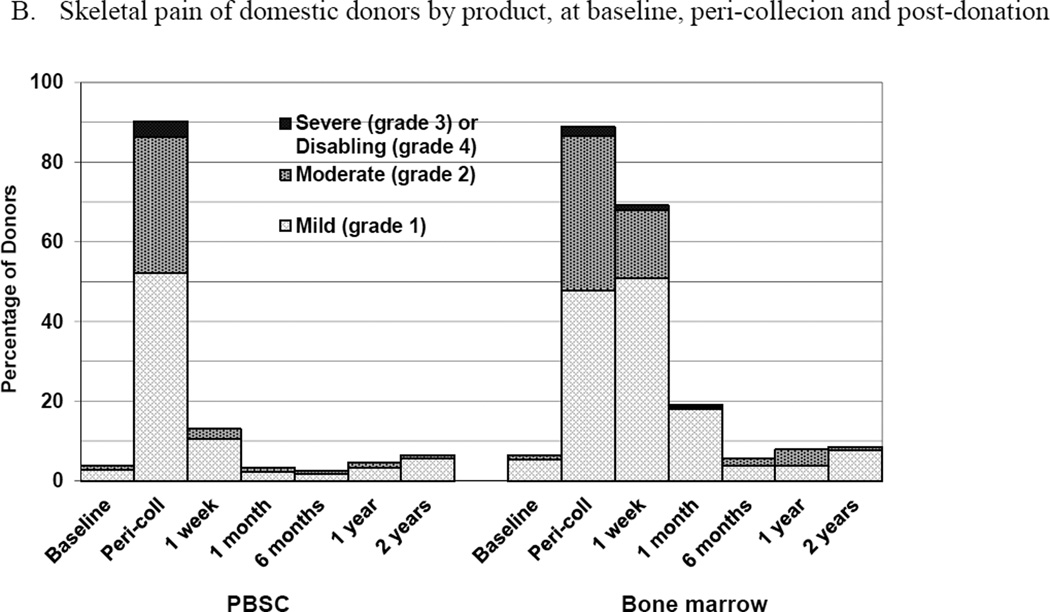
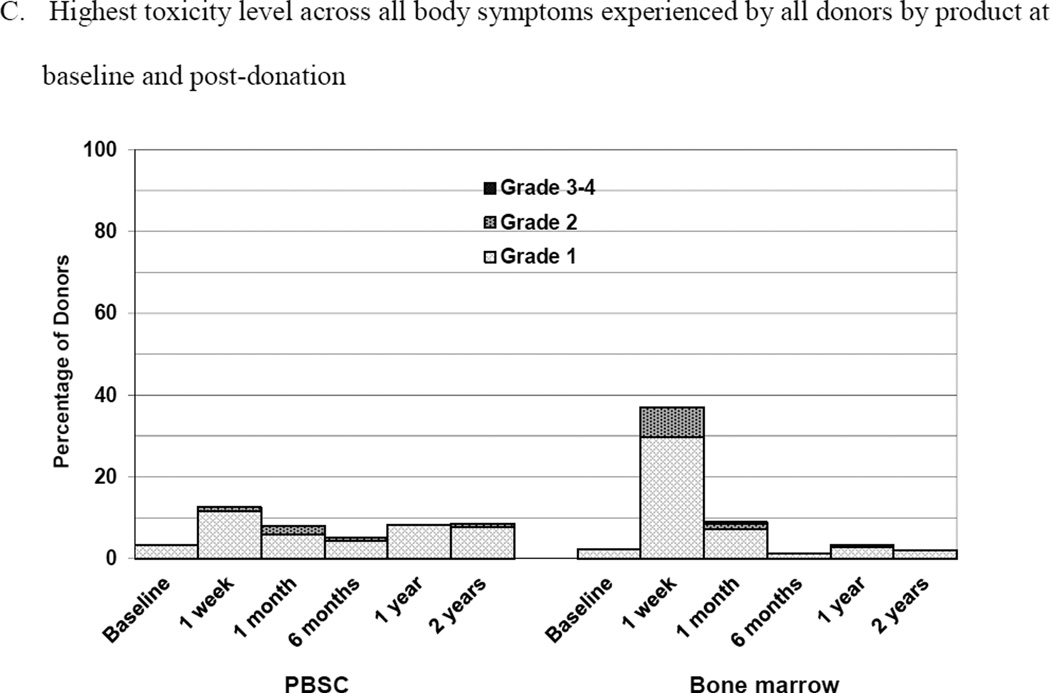
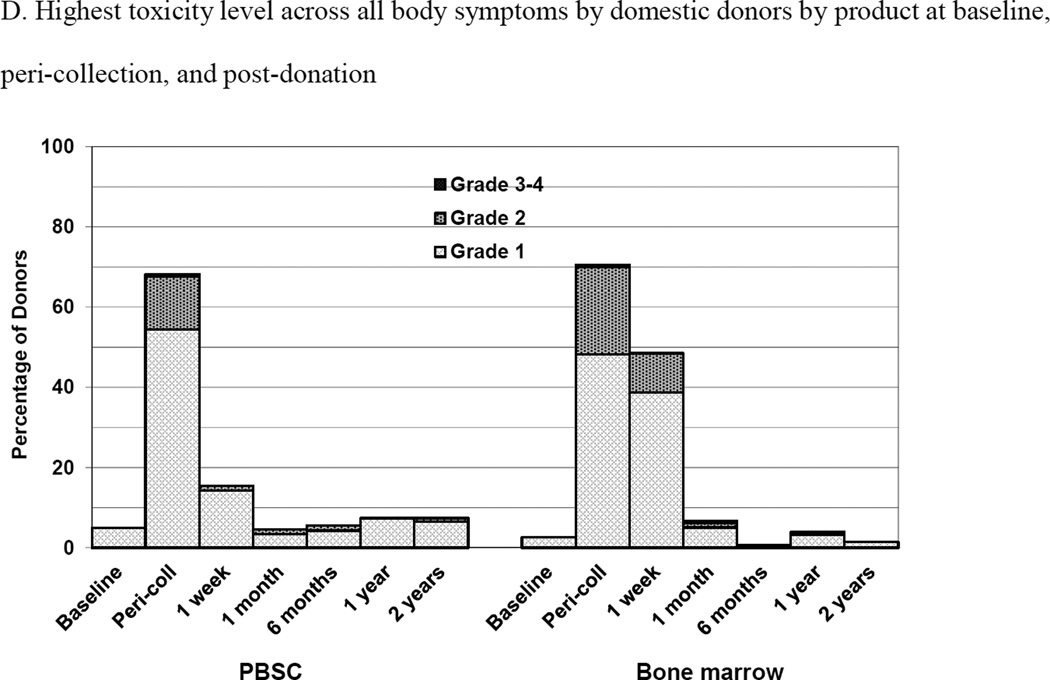
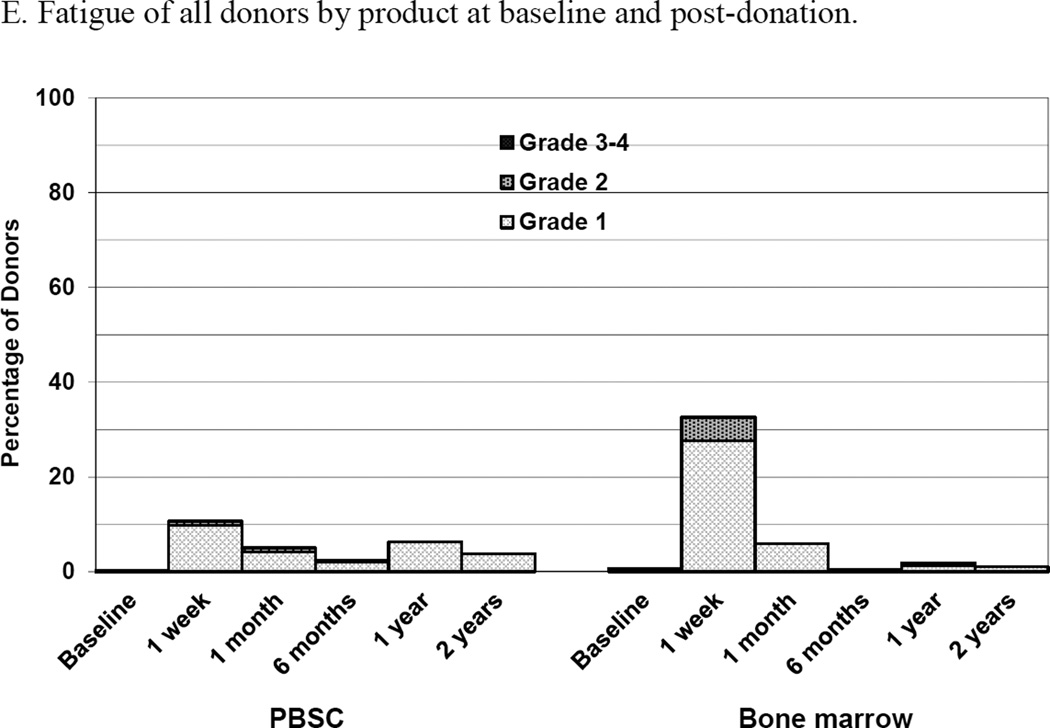
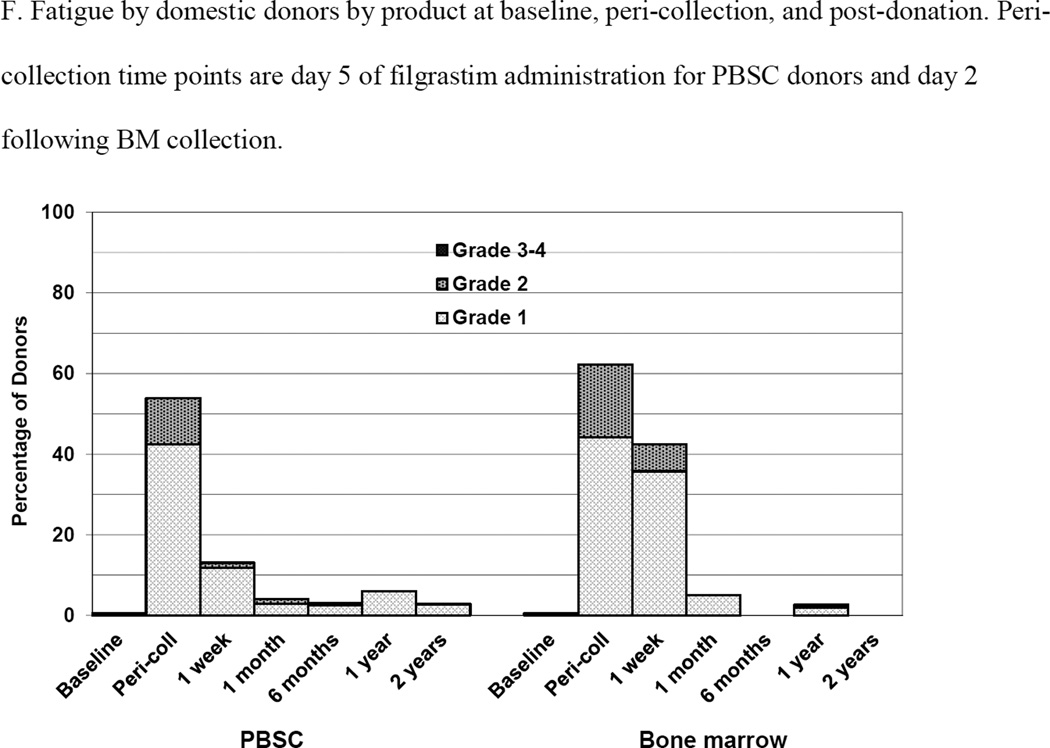
Figure 2.
Skeletal pain and body symptoms by donation of peripheral blood stem cells (PBSC) or bone marrow. (A) Skeletal pain experienced by all donors by product at baseline and post-donation. (B) Skeletal pain of domestic donors by product at baseline, peri-collection and post-donation. (C) Highest toxicity level across all body symptoms experienced by all donors by product at baseline and post-donation. (D) Highest toxicity level across all body symptoms by domestic donors by product at baseline, peri-collection, and post-donation. (E) Fatigue of all donors product at baseline and post-donation. (F) Fatigue by domestic donors by product at baseline, peri-collection, and post-donation. Peri-collection time points are day 5 of filgrastim administration for PBSC donors and day 2 following BM collection.