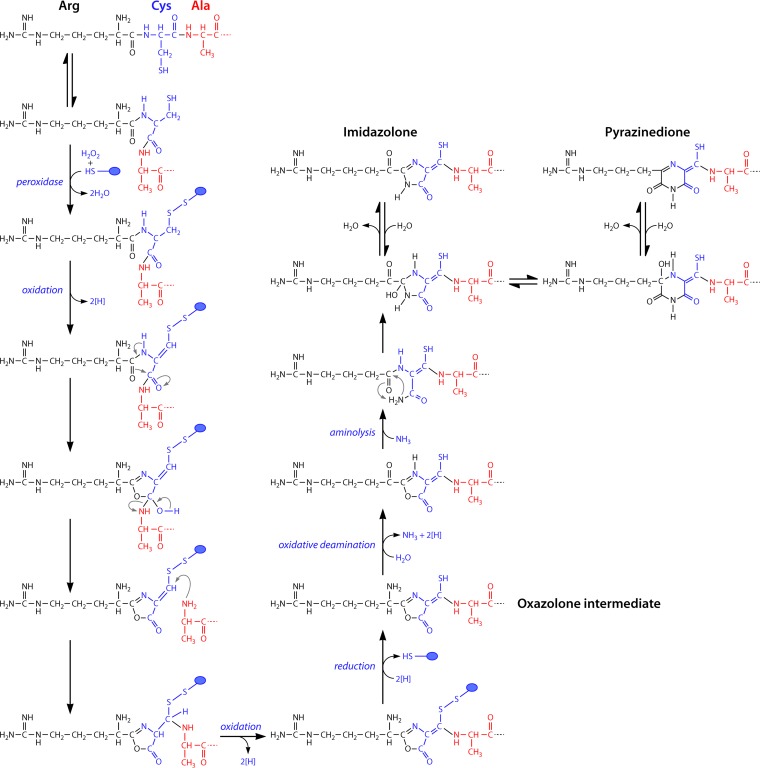
FIG 6.
Proposed reaction schemes for biosynthesis of the oxazoline rings with associated thioamide groups via a tandem two-step sequence of peroxidation and dehydration reactions. Cysteine thiols are likely protected against oxidation, possibly as disulfides involving one of the proteins of the mb gene cluster (blue circles). For imidazolone and pyrazinedione ring formation, oxazolone rings are modified via a transamination/deamination step followed by an aminolysis step to open the oxazolone ring followed by ring formation and dehydration.