Abstract
The Gartner curve for regenerative and stem cell therapeutics is currently climbing out of the “trough of disillusionment” and into the “slope of enlightenment”. Understanding that the early years of stem cell therapy relied on the model of embryonic stem cells (ESCs), and then moved into a period of the overhype of induced pluripotent stem cells (iPSCs), instead of using the model of 40 years of success, i.e. adult stem cells used in bone marrow transplants, the field of stem cell therapy has languished for years, trying to move beyond the early and poorly understood success of bone marrow transplants. Recent studies in the lab and clinic show that adult stem cells of various types, and the molecules that they release, avoid the issues associated with ESCs and iPSCs and lead to better therapeutic outcomes and into the slope of enlightenment.
The Gartner Curve
When an innovation arises that captures the public’s imagination, many will embrace the innovation with high expectations (peak of inflated expectations). This “next big thing” promises to transform the people or the companies that adopt it. Then, at the outset, when the innovation fails to deliver as promised, the herd of devotees starts to bail out (trough of disillusionment). At this point, investments can be wasted, stock prices plunge, and disillusionment in the innovation sets in. As Fenn and Raskino1 explain, the innovation process does not have to be this way. Instead, if we understand the hype curve (Figure 1), we can properly set our expectations and better understand what to invest in, and when to make that investment, yielding success for the long-term.
Figure 1.
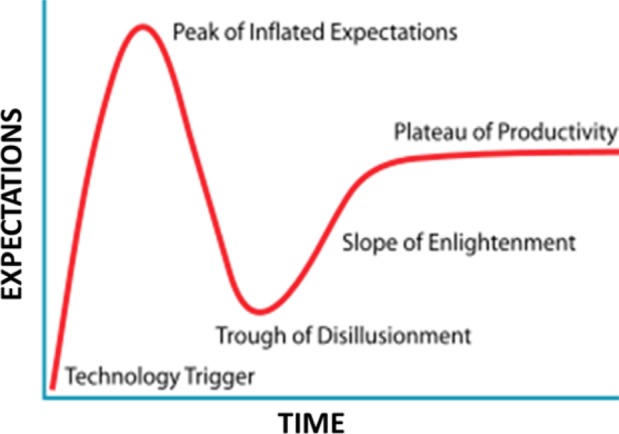
Hype curve for regenerative therapeutics.1
Gartner Curve for Stem Cells in Therapeutics
The technology trigger in the stem cell space was first embryonic stem cells (ESCs), followed by induced pluripotent stem cells (iPSCs). The old model of adult stem cells (ASCs) as used in bone marrow transplants failed to act as a trigger in the business of therapeutic development, and was viewed as uninteresting by most. Instead, the peak of inflated expectations was triggered by ESCs and iPSCs, because of the hype that these types of stem cells could transform into nearly any somatic cell type in the body. The many difficulties involved in working with these two stem cell types was greatly overlooked, and their therapeutic benefit as compared to adult stem cells was greatly exaggerated. iPSCs even suffer from genetic and epigenetic reprogramming errors,2,3 and directly reprogrammed cells revert to an unwanted aged phenotype when transformed to another somatic cell type.4 Indeed, for many diseases or conditions, the adult stem cells, as evidenced early on by the bone marrow stem cells, have been the most efficacious stem cell therapeutic. Why have the adult cells proven to be superior therapeutics? The answer is simple: adult stem cells exist in our adult bodies to maintain and heal our tissues, not ESCs. The ESCs exist only during the first 5 days postfertilization of the egg,5 and serve to drive early development of the embryo. The endogenous therapeutic actions of the adult body are because of adult stem cells, not embryonic stem cells. And specific types of adult stem cells in specific tissues carry out the healing processes.6 In the case of bone marrow transplants, bone marrow stem cells were used for the transplant. Likewise, if we want to heal the nervous system, for example, then we need to use adult stem cells that are resident in the nervous system for developing the therapeutic. A “one size fits all” approach, where a single stem cell type is derived from fat tissue to treat all diseases is a flawed strategy. For example, human liver stem/progenitor cells (ADHLSCs) and hepatic stellate cells (HSCs) are both derived from the liver, but release a different set of molecules.7 And, the same stem cell type under different conditions will release a different pool of molecules.8 Thus, even in one tissue type, multiple stem cell phenotypes exist.
How do the ASCs work, especially as compared to ESCs? It is the molecules. The molecules known to be secreted by ASCs include hundreds of proteins,9 lipids,10 and microRNA.11 As an example, a microRNA is shown in Figure 2. Whether we look at experimental models in the laboratory, or clinical therapeutics for humans, the mechanism of action for most stem cell therapeutics is their release of a multitude of molecules.6,12 So, instead of injecting cells, which may die or become dysfunctional, or not perfuse into the tissue where the cells are needed to induce repair, we can simply administer the molecules that the stem cells make. Thus, the correct stem cell types for the particular tissue to be treated can be optimally grown and stimulated in the laboratory, their molecules can be collected, and then those molecules can be administered to the patient in a known dosing regimen, both in space and in time. This is known as “stem cell therapy without the cells”,12 and represents the next generation of stem cell science as a “systems therapeutic”.13 The concept of the “systems therapeutic” is that a disease state or condition is caused by defects in many biological pathways, and that all of the defective pathways should be treated. This is in contradistinction to the traditional small molecule drug development model where one molecule that targets one pathway is used to ameliorate the condition. Thus, the ASCs, through their action of releasing many molecule types, act as a systems therapeutic.13 Further, because the molecules are naturally packaged into exosomes by the stem cells, the molecules are protected and delivered to the target tissue together in space and in time, yielding a collective, synergistic effect of the molecules. Interestingly, nearly 25% of human proteins are known to be secreted by exosomes.14
Figure 2.

MicroRNA: Example of the molecules released from adult stem cells.
Mature microRNAs (shown in red) are 21- to 23-nucleotide-long, single-stranded RNAs processed (in the cytoplasm) from longer transcripts, called pre-miRNA (stem loop RNA). Pre-miRNA have a long, distinctive duplex containing several mispairs forming an apical loop of variable size. Thousands of miRNA have been identified in humans.15
Gartner Curve for Stem Cells in Medicine
Physicians have known well the value of bone marrow transplants for the treatment of leukemia and multiple myeloma. With the later understanding that the success of bone marrow transplants was largely attributed to adult stem cells,16 coupled with the newly found knowledge that adult stem cells could be found in the blood and fat tissues, the hype arose that a simple transplantation of any tissue containing ASCs could be used to treat almost any condition. As such, clinics sprang up around the world with the promise to treat diseases from cancer to MS to joint diseases using simple medical procedures relying on autologous ASCs derived from fat and blood.
Medicine can be practiced with the aid of approved drugs, and/or with the use of medical procedures. Sometimes the distinction between a drug and a medical procedure becomes blurred. This has been the case for autologous stem cell procedures. The FDA considers autologous stem cell therapy to be a drug when the stem cells extracted from the patient have been more than “minimally manipulated”. This severely limits the ability to use autologous stem cells in the medical practice. In effect what happens in the medical procedure using autologous stem cells is that fat or blood is extracted from the patient, and then simply centrifuged to develop a pellet of tissue that presumably contains live stem cells. The resulting pellet can then be used for transplantation purposes only if the cells in the pellet have not been manipulated in any fashion, including procedures to expand the cell count. Once a manipulation is done, the FDA considers the cells to be a drug, falling out of the jurisdiction of a medical procedure and into the jurisdiction of the Agency’s drug development regulations. With regulations in place on medical treatments, only simple stem cell procedures, without cellular manipulation, could be performed by the physician as a medical procedure. The simple procedures allowed in medicine even meant that the adult stem cells could not be identified, or even evaluated for their numbers or viability. Beyond the difficulty of knowing whether you had viable stem cells for transplant, the physician had no way of understanding whether the stem cells, once they were transplanted, would remain viable in the body and perfuse to the tissue in need of reparation. Clearly, the adult stem cell procedure practiced by the physician as a medical procedure and not as a drug meant that an autologous stem cell procedure had a high probability of failure. This is evidenced by the high profile individuals who have had such procedures, for joint repair as an example, with little or no benefit.17 Like therapeutic development with stem cells, as described in the aforementioned section, medical procedures with stem cells currently exist in the “trough of disillusionment”.
The SRM Model
Using the stem cell released molecules (SRM), also known as secretome, as the therapeutic is the next generation of stem cell science, from which a generation of stem cell therapeutics will be produced that are grounded in the well documented success of using adult stem cells, and a business model that follows well accepted practices in the pharmaceutical industry. Let us explore how the science, technology, and business model of using the SRM moves well beyond the cellular models used in the past. The new SRM technology recognizes that ASCs exist in the body as a multitude of different phenotypes, with tissue specific phenotypes serving particular functions resident in the skin versus the brain versus the eye, for example. Each phenotype releases its distinct collection of molecules.6
Consider how the secretome technology is different from that of cell-based therapeutics. First, the molecules (SRM) are in no way limited by the WARF patents that have been rigorously enforced by the owners of the patents and has led to delays in the development of ESC-based therapeutics. The SRM technology is adult stem cell based, and therefore avoids the early ESC patent wall established by WARF. The now conflicting patents that exist based on identifying stem cell types with a limited array of markers is nonconcerning to the SRM technology. That is, conflicting patents have been awarded for identifying stem cell types based on markers that were not unique and led to different patents being awarded that had labeled the same stem cell types. SRM-based therapy uses the molecules only, and not the contested cells.
Because nonembryonic stem cells are used, the legal, moral, and religious problems associated with embryonic stem cells are avoided. Further, adult cells, unlike embryonic stem cells, are noncancerous. Embryonic stem cells can form teratomas, a type of cancer tumor. And because no cells are used in SRM, the concern of cancer is even further diminished and an immune response is avoided. Moreover, the molecules are protected from the immune system, especially given their packing within the immunoprivileged exosome.18 SRM also avoids the problem of cells persisting in the body. Unlike cell therapy, where an AE can develop and the cells causing the AE stay in the body, the SRM dissipates and administration can be stopped.
Another advantage of SRM is that one product works for many patients. Instead of autologous procedures where one product is used for only one patient, a costly and cumbersome methodology, SRM can be used on all patients. Finally, SRM offers an easier development and commercialization pathway than that for cells. Molecules are easily produced in a laboratory, stored (maybe lyophilized), and delivered to the patient in a convenient form of administration, whereas cells are difficult to keep alive during storage, during transportation to the patient, and then when administered to the patient. Working with the cells is a much more unwieldly and expensive process than that for working with the molecules.
Conclusions
The stem cell released molecules from multiple adult stem cell types, packaged into naturally produced exosomes, offer a well proven means to heal the body, and provide a therapeutic that is more efficacious, easier, and less expensive to develop and deliver to the patient, and they fit better into the current drug development model than do cell-based therapeutics.
The authors declare the following competing financial interest(s): Part owner of BioRegenerative Sciences, Inc, a stem cell therapeutics company.
References
- Fenn J.; Raskino M.. Mastering the Hype Cycle: How to Adopt the Right Innovation at the Right Time; Harvard University Press: Cambridge, MA, 2008. [Google Scholar]
- Gore A.; Li Z.; Fung H. L.; Young J. E.; Agarwal S.; Antosiewicz-Bourget J.; Canto I.; Giorgetti A.; Israel M. A.; Kiskinis E.; Lee J. H.; Loh Y. H.; Manos P. D.; Montserrat N.; Panopoulos A. D.; Ruiz S.; Wilbert M. L.; Yu J.; Kirkness E. F.; Izpisua Belmonte J. C.; Rossi D. J.; Thomson J. A.; Eggan K.; Daley G. Q.; Goldstein L. S.; Zhang K. Somatic Coding Mutations in Human Induced Pluripotent Stem Cells. Nature 2011, 471, 63–67. 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann U.; Wu G.; Marthaler A. G.; Schöler H. R.; Tapia N. Epigenetic Aberrations Are Not Specific to Transcription Factor-Mediated Reprogramming. Stem Cell Rep. 2016, 6, 35–43. 10.1016/j.stemcr.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J.; Paquola A. C. M.; Ku M.; Hatch E.; Böhnke L.; Ladjevardi S.; McGrath S.; Campbell B.; Lee H.; Herdy J. R.; Gonçalves J. Y.; Toda T.; Kim Y.; Winkler J.; Yao J.; Hetzer M. W.; Gage F. H. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015, 17, 705–718. 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman G.; De Rycke M.; Sermon K.; Liebaers I.; Van de Velde H. Markers that Define Stemness in ESC are Unable to Identify the Totipotent Cells in Human Preimplantation Embryos. Hum. Reprod. 2009, 24, 63–70. 10.1093/humrep/den351. [DOI] [PubMed] [Google Scholar]
- Maguire G.; Friedman P. Systems Biology Approach to Developing S2RM-Based “Systems Therapeutics” and Naturally Induced Pluripotent Stem Cells. World J. Stem Cells 2015, 7, 745–756. 10.4252/wjsc.v7.i4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardis S.; Lombard C.; Evraerts J.; El Taghdouini A.; Rosseels V.; Sancho-Bru P.; Lozano J. J.; Grunsven L.; Sokal E.; Najimi M. Gene Expression Profiling and Secretome Analysis Differentiate Adult-Derived Human Liver Stem/Progenitor Cells and Human Hepatic Stellate Cells. PLoS One 2014, 9 (1), e86137. 10.1371/journal.pone.0086137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J.; Kim J.; Kim M. Y.; Bae Y. S.; Ryu S. H.; Lee T. G.; Kim J. H. Proteomic Analysis of Tumor Necrosis Factor-α-Induced Secretome of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Proteome Res. 2010, 9, 1754–1762. 10.1021/pr900898n. [DOI] [PubMed] [Google Scholar]
- Sze S. K.; de Kleijn D. P.; Lai R. C.; Khia Way Tan E.; Zhao H.; Yeo K. S.; Low T. Y.; Lian Q.; Lee C. N.; Mitchell W.; El Oakley R. M.; Lim S.K. Elucidating the Secretion Proteome of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells. Mol. Cell. Proteomics 2007, 6, 1680–1689. 10.1074/mcp.M600393-MCP200. [DOI] [PubMed] [Google Scholar]
- Ranganath S. H.; Levy O.; Inamdar M. S.; Karp J. M. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell. 2012, 10, 244–258. 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collino F.; Deregibus M. C.; Bruno S.; Sterpone L.; Aghemo G.; Viltono L.; Tetta C.; Camussi G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of miRNAs. PLoS One 2010, 5 (7), e11803. 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G. Stem Cell Therapy Without the Cells. Commun. Integr. Biol. 2013, 6 (6), E26631. 10.4161/cib.26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G. Systems Biology Approach to Developing ″Systems Therapeutics.″. ACS Med. Chem. Lett. 2014, 5, 453–455. 10.1021/ml5000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthikumar S.; Chisanga D.; Ariyaratne D.; Saffar H. A.; Anand S.; Zhao K.; Samuel M.; Pathan M.; Jois M.; Chilamkurti N.; Gangoda L.; Mathivanan S. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J.; Heimfeld S.; Weissman I. L. Purification and Characterization of Mouse Hematopoietic Stem Cells. Science 1988, 241, 58–62. 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Franklin D. A.Dangerous Game: Some Athletes Risk Untested Stem Cell Treatments. Sci. Am.. 2013, 308, 30. 10.1038/scientificamerican0513-30 (http://www.scientificamerican.com/article/a-dangerous-game-athletes-risk-untested-stem-cell-treatments/) [DOI] [PubMed] [Google Scholar]
- Maguire G.Exosomes: Smart Nanospheres for Drug Delivery Naturally Produced by Stem Cells. In Fabrication and Self-Assembly of Nanobiomaterials; Elsevier: Amsterdam, 2016; pp 179–209. [Google Scholar]
- Londin E.; Loher P.; Telonis A. G.; Quann K.; Clark P.; Jing Y.; Hatzimichael E.; Kirino Y.; Honda S.; Lally M.; Ramratnam B.; Comstock C. E. S.; Knudsen K. E.; Gomella L.; Spaeth G. L.; Hark L.; Katz L. J.; Witkiewicz A.; Rostami A.; Jimenez S. A.; Hollingsworth M. A.; Yeh J. J.; Shaw C. A.; McKenzie S. E.; Bray P.; Nelson P. T.; Zupo S.; Van Roosbroeck K.; Keating M. J.; Calin G. A.; Yeo C.; Jimbo M.; Cozzitorto J.; Brody J. R.; Delgrosso K.; Mattick J. S.; Fortina P.; Rigoutsosa I. Analysis of 13 Cell Types Reveals Evidence for the Expression of Numerous Novel Primate- and Tissue-Specific microRNAs. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 1106–1115. 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]


