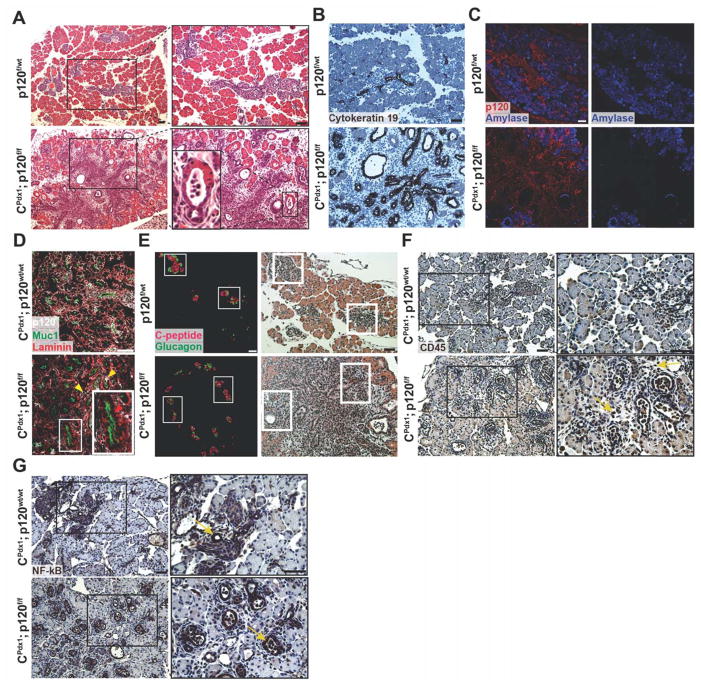
Figure 2. Characterization of p120 catenin deletion in neonatal P0 mice.
(A) Hematoxylin and eosin staining showed disruption of pancreatic tissue architecture in homozygous p120f/f animals compared to wild-type controls. A magnified image of an abnormal transitional structure is shown. (B) Immunohistochemistry demonstrated that expanded ductal epithelium in homozygous p120f/f pancreases is Cytokeratin 19 positive. (C) Amylase immunofluorescence showed aberrant acinar cell differentiation in CPdx1; p120f/f pancreases. (D) Immunofluorescent imaging documented epithelial-specific p120 catenin deletion. In the CPdx1; p120f/f figure, the top right yellow arrow points to a rare p120 catenin-expressing epithelial cell. The left yellow arrow points to a p120 catenin-expressing non-epithelial cell. The red arrow in the inset shows epithelia in CPdx1; p120f/f pancreas that lack p120 catenin. Note that p120 catenin staining is nearly completely absent in CPdx1; p120f/f epithelial pancreas, with very few cells mosaic for p120 catenin expression. Muc1 and Laminin provide clear boundaries for distinction of apical and basal regions of the pancreatic epithelium. (E) Immunofluorescence of C-peptide and Glucagon demonstrated abnormal islet distribution in homozygous p120f/f pancreas. Identical sections were stained with hematoxylin and eosin to demonstrate tissue context, and white boxes show corresponding islets. (F) CD45 immunohistochemistry revealed the recruitment of an inflammatory infiltrate in CPdx1; p120f/f pancreas, but not in wild-type controls. In the CPdx1; p120f/f bottom panel, yellow arrows point to CD45+ cells. (G) NF-kB signaling was detected in the ductal epithelium of both wild-type and homozygous p120f/f pancreas, which is indicated by yellow arrows. Scale bars are 50μm.