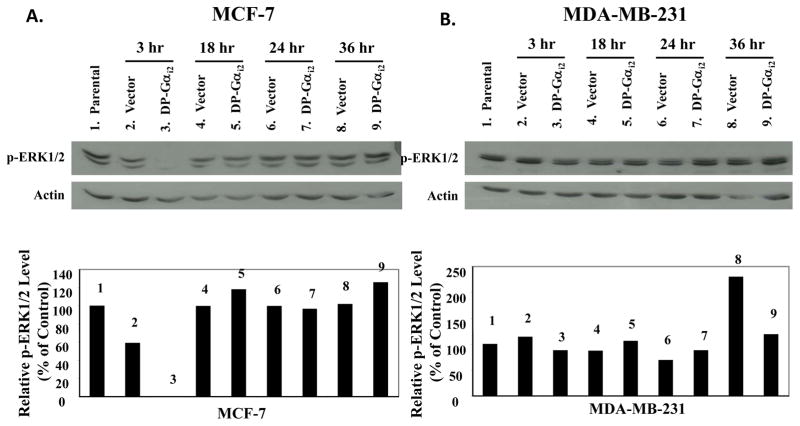
Figure 5. Effects of the dominant-positive Gαi2 protein on the activation of ERK1/2 in MCF-7 and MDA-MB-231 human breast cancer cells.
(A) MCF-7 and (B) MDA-MB-231 breast cancer cells were plated onto 10-cm Petri dishes at a density of 1.2 × 106 cells/dish in RPMI-1640 supplemented with 10% FBS. Twenty-four hours after seeding, cells were sham transfected (Parental), or transfected with 600 ng/dish of pcDNA3.1 (Vector) or dominant-positive Gαi2 protein (DP-Gαi2) plasmid for 6 hours and then harvested at 3, 18, 24, and 36 hours following transfection. Whole cell lysates were extracted from MCF-7 and MDA-MB-231 cells. Fifty micrograms of total cellular protein was electrophoretically separated on 10% SDS polyacrylamide gel, and transferred onto nitrocellulose membrane. The phosphorylation of ERK1/2 was examined by Western blot analyses using anti-phospho-p44/42 MAPK (Thr183/Tyr185) antibody, at a 1:1000 dilution. The same blots were stripped and reprobed with anti-actin antibody. Western blot signal intensities were measured by phosphoimage densitometry and then normalized to the actin levels (bottom graph). Results were expressed as relative arbitrary units with a value of 100% for the parental control groups. This figure is a Western blot analysis of ERK1/2 phosphorylation in MCF-7 and MDA-MB-231 breast cancer cells, and it is representative of 3 independent experiments.