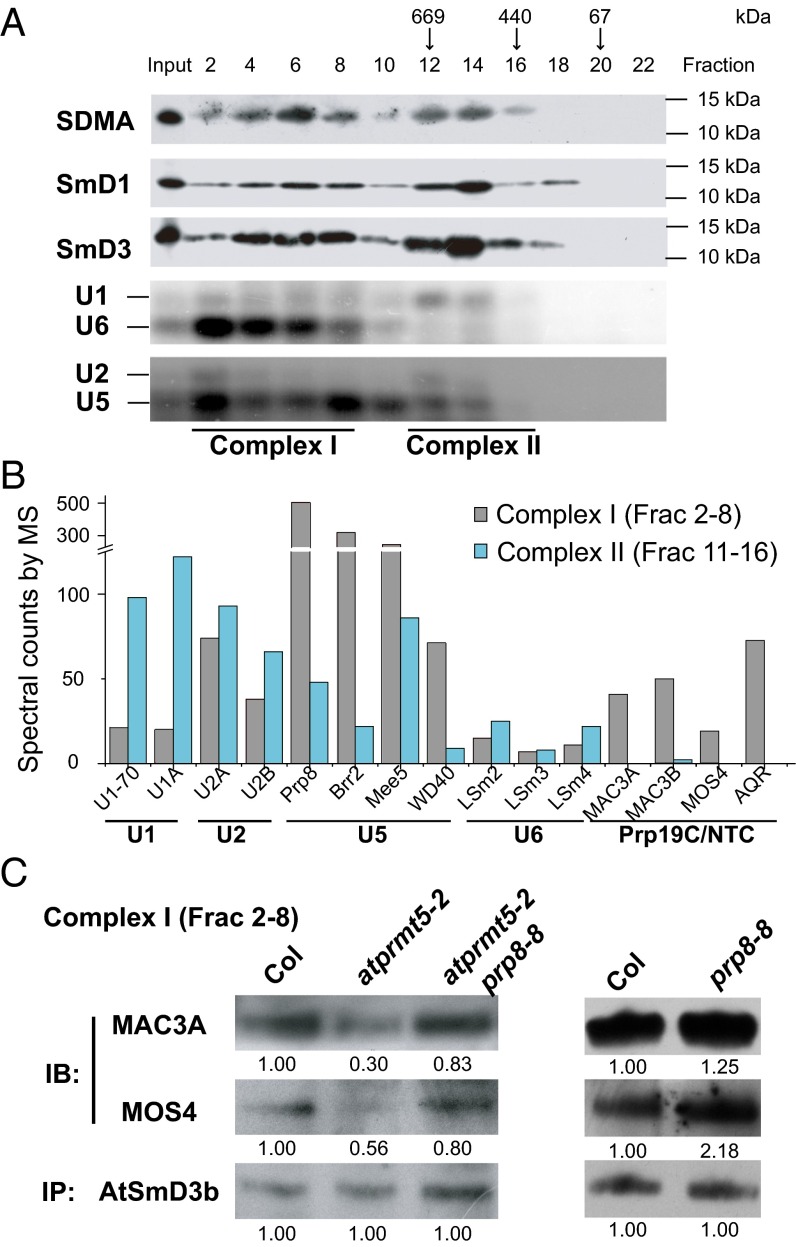
Fig. 5.
Decreased recruitment of Prp19C/NTC to the spliceosome in atprmt5 mutants. (A) The elution profiles of symmetric dimethylated AtSmD1 and AtSmD3 proteins. Cell lysates from Col were fractionated by gel filtration chromatography as described in Materials and Methods and were immunoblotted to visualize symmetric dimethylated AtSmD1 and AtSmD3. The molecular weights of the calibration standards and their elution positions are indicated. (B) The distribution of the spliceosomal proteins in gel filtration chromatography. Fractions 2–8 and 11–16 were collected and immunoprecipitated with SDMA antibody, and then the associated proteins were identified by MS. The spectral counts of U snRNPs and Prp19C/NTC are shown in the histogram. (C) The interaction status between AtSmD3b and Prp19C/NTC in complex I. Fractions 2–8 were collected from Col, atprmt5-2, atprmt5-2 prp8-8, and prp8-8, and AtSmD3b was immunoprecipitated by anti-AtSmD3b polyclonal antibody, and then immunoblotting was performed with anti-MAC3A and anti-MOS4 polyclonal antibodies for Prp19C/NTC and anti-AtSmD3b antibody for loading controls.