Significance
The plastron, the order-defining skeletal structure for turtles, provides a bony exoskeleton for the ventral side of the turtle. We provide here the first molecular analysis of plastron bone formation. We show that plastron bone morphogenesis in the ventral mesenchyme employs a program of bone formation that usually characterizes the vertebrate face and skull. The plastron bones, however, have a preliminary step that is not included in head formation: They must suppress the usual chondrogenic programs that would create the sternum cartilage. We suggest that the early osteogenic fate adopted by the ventral mesenchyme prevents the chondrogenic sternal development in turtles and that this was a critical step in forming the ossification centers for this new type of vertebrate structure.
Keywords: turtle, plastron, osteogenesis, development
Abstract
The dorsal and ventral aspects of the turtle shell, the carapace and the plastron, are developmentally different entities. The carapace contains axial endochondral skeletal elements and exoskeletal dermal bones. The exoskeletal plastron is found in all extant and extinct species of crown turtles found to date and is synaptomorphic of the order Testudines. However, paleontological reconstructed transition forms lack a fully developed carapace and show a progression of bony elements ancestral to the plastron. To understand the evolutionary development of the plastron, it is essential to know how it has formed. Here we studied the molecular development and patterning of plastron bones in a cryptodire turtle Trachemys scripta. We show that plastron development begins at developmental stage 15 when osteochondrogenic mesenchyme forms condensates for each plastron bone at the lateral edges of the ventral mesenchyme. These condensations commit to an osteogenic identity and suppress chondrogenesis. Their development overlaps with that of sternal cartilage development in chicks and mice. Thus, we suggest that in turtles, the sternal morphogenesis is prevented in the ventral mesenchyme by the concomitant induction of osteogenesis and the suppression of chondrogenesis. The osteogenic subroutines later direct the growth and patterning of plastron bones in an autonomous manner. The initiation of plastron bone development coincides with that of carapacial ridge formation, suggesting that the development of dorsal and ventral shells are coordinated from the start and that adopting an osteogenesis-inducing and chondrogenesis-suppressing cell fate in the ventral mesenchyme has permitted turtles to develop their order-specific ventral morphology.
The ventrum of turtles is covered by a protective shell, the plastron, composed of bony plates that are themselves covered by keratinous scutes. The plastron is thought to be the oldest part of turtle’s shell as the earliest known turtles in the fossil record to date—Odontochelys and Pappachelys—had plastron-like ventral bones, but only a partial carapace (1, 2). Most modern turtles have nine bones in the plastron that develop within the ventral mesenchyme in between the ectodermal scutes and the visceral organs (Fig. 1). The anterior of the plastron contains a medial rostral entoplastron bone and two lateral rostral epiplastron bones that will grow and suture together to form an anterior bone plate. The entoplastron is thought to be derived from the interclavicle bone, whereas the paired epiplastra are thought to be homologous to the clavicles (3–5). In the primitive turtle Proganochelys a dorsal process extending from the epiplastron bone to the carapace acted as a clavicle but in modern turtles this dorsal process is missing. Instead, a modified acromion process of the scapula blade provides contact between the carapace and the entoplastron (6). Three pairs of lateral plastron bones—the centrally located hyoplastra and hypoplastra and the posterior xiphiplastron bones—are thought to be derived from the paired gastralia seen as floating ventral ribs in numerous reptiles groups. This position is made stronger by the paired gastralia seen forming a plastron-like structure in the stem turtle Pappochelys (1). The posterior ends of the hyoplastron bones, together with the anterior ends of hypoplastra, form an umbilical fontanel at the midline of the plastron. Moreover, processes extend dorsally from the hyoplastron and hypoplastron to form a bridge that connects the plastron with the ribs and the carapace. In some turtles (especially ancient lineages), a further set of paired plastron bones, the mesoplastra, lie between the hyoplastra and hypoplastra (7).
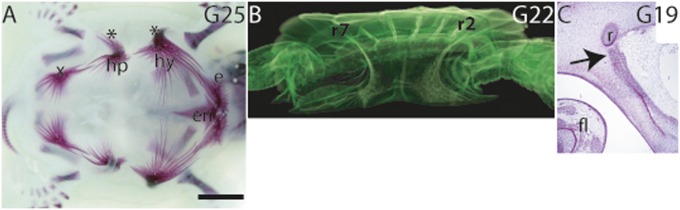
Fig. 1.
The plastron of the hard-shelled turtle T. scripta. (A) Alizarin red stained embryonic plastron at G25. (B) At G22, the bridge bone from the hyoplastron extended underneath the second rib (r2), and the tip of the bridge bone grew past the rib anteriorly. The bridge extension of the hypoplastron had grown underneath the seventh rib (r7), and the tip of the bridge bone extended past the rib posteriorly. Movie S1 demonstrates the bridge extensions. (C) Hematoxylin–eosin-stained sagittal section showing an osteogenic front (arrow) of a hyoplastron bridge bone adjacent to a rib (r) at G19. e, epiplastron; en, entoplastron; fl, forelimb; hp, hypoplastron; hy, hyoplastron; x, xiphiplastron; *, bridge extensions of hyo- and hypoplastron;. (Scale bar in A, ∼0.5 cm.)
Although the anatomy of plastron bones has been known for centuries, and the homology of these bones to the skeletal structures of other reptilian clades has been debated almost as long (3, 4, 6, 8), we still know very little about how these intramembranous bones arise within the ventral mesenchyme and why cartilage does not form in this region. Both bone and cartilage arise from mesenchymal condensations formed by osteochodrogenic progenitor cells, which are able to differentiate either to chondrocytes or osteoblasts depending on their molecular regulation via key transcription factors and signaling pathways (9). This dual potential in osteochondroprogenitor cells is largely due to coexpression of a chondrogenic transcription factor Sox9 and an osteochondrogenic transcription factor Runx2. For osteochondroprogenitor cells to commit to an osteogenic path, the chondrogenic potential must be suppressed; this happens through the inhibition of expression of Sox9, while inducing or maintaining that of Runx2 (10, 11). Coexpression of the transcription factor Twist with Runx2 in osteoprogenitor cells maintains these cells in a proliferative, undifferentiated state—Twist directly binds the DNA-binding domain of Runx2 to inhibit its function (12, 13). Once expression of Twist is switched off, Runx2 is able to induce expression of Osterix (Osx) (14). This molecular regulation ensures osteogenic differentiation and maturation. Commitment to an osteogenic cell lineage also requires the sequential activation of Hedgehog and canonical Wnt/β-catenin signaling in osteoprogenitor cells (15–18). Other critical regulatory systems in intramembranous bone formation include the BMP-induced transcription factor Msx2, which promotes the proliferation of preosteoblasts, while suppressing differentiation (19–21).
The plastron bones appear to have taken over the role of the ventral portion of the ribs and the sternum that are missing in turtles. In most vertebrates, the ribs develop from the somitic mesenchyme within the primaxial domain of the embryo. As they expand, the ribs cross the lateral somitic frontier and enter into the abaxial domain of the embryo (22). The sternum develops fully in the lateral plate mesenchyme. In turtles, the rib development is affected by the turtle-specific signaling/organizing center called the carapacial ridge (23), and the ribs remain as primaxial structures that do not cross the lateral somitic frontier (24–27). In birds and mice, the sternum develops in the lateral plate mesenchyme as paired rod-like cartilage anlagen under the posterior end of the wing/forelimb bud, extending some distance posteriorly at the lateral edge of the ventral mesenchyme. The sternal cartilage anlagen develop independently of the ribs or clavicles, and they migrate toward the ventral midline where they eventually fuse (28–31). The sternal anlagen begin as mesenchymal condensations that express Runx1 and Runx2. These transcription factors cooperate to induce the expression of Sox5 and Sox6. Sox5 and Sox6 initiate chondrogenesis in the condensed cells and subsequently lead to the induction of type II collagen expression, a chondrocyte-specific extracellular matrix component (31, 32). When only Runx2 expression is up-regulated in the lateral plate mesenchyme before sternal morphogenesis, it interferes with chondrogenesis and promotes formation of ectopic intramembranous bone in the ventral mesenchyme (32).
Reptilian gastralia, the homologs of the posterior plastron bones, develop similarly to plastron bones in the ventral mesenchyme through intramembranous ossification. Despite their apparent similarities, they are not equal. Each gastralial bone consists of a lateral and a medial bone element that articulate with each other, and gastralial bones do not form bone spicules. Whereas plastron bones start to mineralize from the periphery of the ventrum in a slight anterior-to-posterior preference (33), gastralia mineralize in a posterior-to-anterior sequence such that the posteriormost row of paired lateral elements of gastralia mineralize first. Once the lateral elements have mineralized, the medial elements begin to mineralize, starting again at the posterior end (34). Other exoskeletal dermal bones found in various reptilian taxa include osteoderms, which differ from plastron bones in that they ossify via the metaplasia of fibrous connective tissue into bone (34). Unlike the developing plastron bones, which develop intramembranously, these osteoderms lack osteoblasts, osteoid, and periosteum (35).
Here we demonstrate that the ventral mesenchyme of the Emydid cryptodire turtle Trachemys scripta elegans is biased to form bone at the expense of cartilage in the same manner as calvarial and facial ectomesenchyme, the tissue most studied for the molecular regulation of intramembranous ossification. In turtles, from developmental Greenbaum stage (G)15 onwards, we observed a series of mesenchymal condensations corresponding to each plastron bone forming within the lateral edges of the ventral mesenchyme below the fore- and hindlimb buds. Each condensation followed the transcriptional code leading to osteogenic lineage commitment; initially Sox9 expressing osteochondrogenic precursor cells suppressed their chondrogenic potential and began to express genes directing osteogenesis. We also show that transient paired mesoplaston osteogenic condensations formed between the hyo- and hypoplastron condensations at the early stages of plastron bone development. Characterization of plastron bone growth demonstrated that the formation of bone spicules is intrinsic to plastron bone development and patterning, and that their growth is driven by the osteogenic signaling. The timing and location of early development and osteogenic commitment of plastron bone condensations seems to overlap with that of paired chondrogenic sternal condensations in birds and mice (28, 30). Thus, we suggest that the order-specific ventral morphology in turtles is created by intrinsic patterning information in ventral mesenchyme that suppresses chondrogenesis and drives osteogenesis.
Results
Osteogenic Fate of Plastron Bones and Ventral Mesenchyme.
Turtle skeleton formation has been studied in multiple species from hard-shelled to soft-shelled turtles and from side-necked (Pleurodira) to hidden-neck turtles (Cryptodira) (33, 36–40). These studies have emphasized the development of the carapacial skeleton, following the order of mineralization of skeletal elements during late embryogenesis and comparing carapace development between different species of turtles. Here we studied plastron bone development in the hard-shelled red-eared pond slider Trachemys scripta from its earliest stages of development. We used X-ray microtomography (μCT) imaging to detect the mesenchymal condensations that will become plastron bones, and phosphotungstic acid (PTA) was used as a contrast stain to show regions of higher tissue density within the turtle embryonic mesenchyme (41). At developmental stage G17, nine definite areas encircling the periphery of the ventral mesenchyme were seen to be denser (i.e., more condensed) than the surrounding mesenchyme (Fig. 2A). The locations of these condensations matched the location of the nine future plastron bones (compare with Fig. 1A). A transverse digital section of a μCT imaged embryo and a hematoxylin–eosin-stained section of the hyoplastron condensation showed extracellular matrix in the center and condensed mesenchymal cells surrounding it, indicating that the developing plastron bones are osteoids at this stage (Fig. 2 B and C). Nothing resembling rod-like sternal cartilage anlagen was seen in μCT imaged samples (Fig. 2A), indicating that sternum development was not initiated in the turtle.
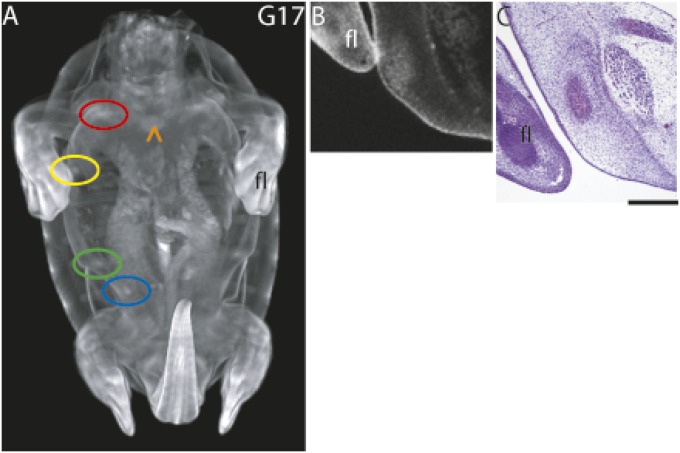
Fig. 2.
Plastron bone condensations. (A) PTA-stained G17 embryo showing nine developing plastron bones. (B) Digital transverse section of hyoplastron shown in A. (C) Hematoxylin–eosin-stained transverse section of forming hyoplastron consisting of an extracellular matrix-filled center surrounded by condensed preosteoblastic cells. Color code: red, epiplastron; yellow, hyoplastron; green, hypoplastron; blue, xiphiplastron. These have pairs on the opposite side. Orange ^ indicates the entoplastron as barely visible linear bar at midline. fl, forelimb. (Scale bar in histological section, ∼0.5 mm.)
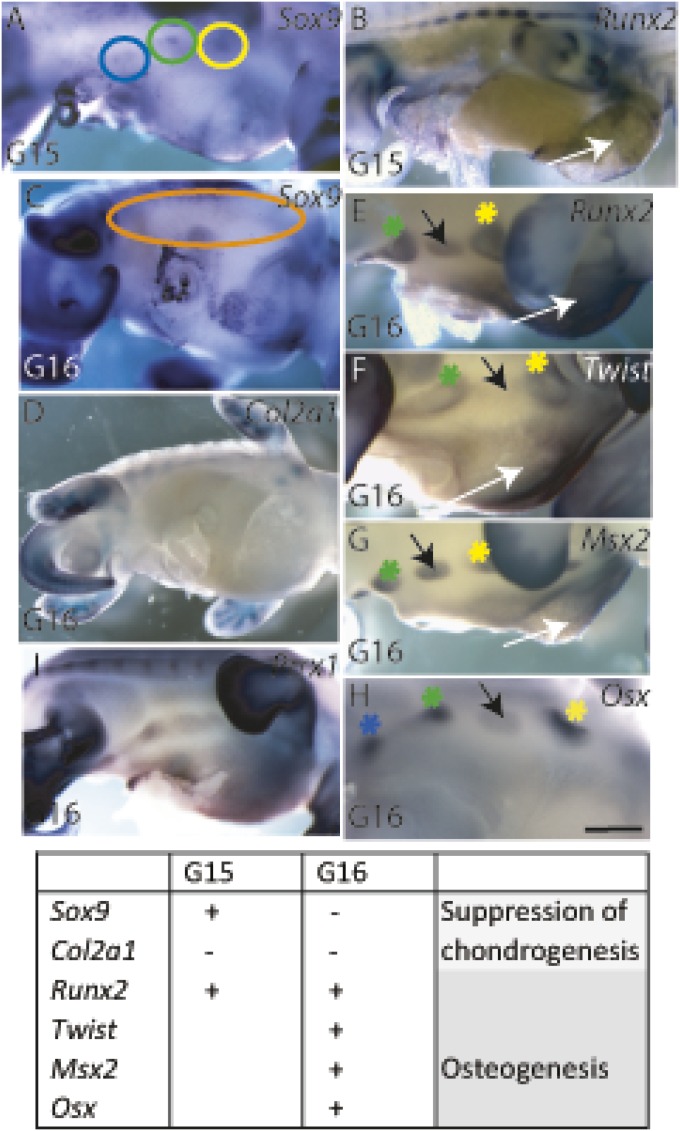
Osteochondrogenic marker gene expression patterns allowed us to establish a developmental timeline and the locations of osteogenesis in the developing plastron. The initiation of plastron bones was seen at G15 when osteochondrogenic mesenchymal condensations for the hyo-, hypo-, and xiphiplastron were expressing Sox9 in distinct regions at lateral edges of ventral mesenchyme between the fore- and hindlimb but were not yet expressing Runx2 (Fig. 3 A and B). By G16, the expression patterns of Sox9 and Runx2 were reversed (Fig. 3 C and D), indicating that chondrogenic potential had been suppressed and the condensations had committed to an osteogenic fate. Lack of chondrocytes was confirmed by lack of collagen II expression (Fig. 3D). Osteogenic plastron bone condensations expressed Runx2 throughout the condensations (Fig. 3E). Twist was expressed at the osteogenic fronts of the condensations and only minimally within them (Fig. 3F). The expression of Msx2 overlapped with that of Runx2 in the developing plastron bones except for the Twist-positive osteogenic fronts (Fig. 3G), and Osx expression was found in the core of each of the developing plastron bones (Fig. 3H). Thus, the plastron bones displayed the same combinations and sequence of osteogenic transcription factors found in any developing intramembranous bones: the centers of the condensations expressed osteogenic factors that drive the osteogenesis toward differentiation (Msx2, Runx2, and Osx), whereas the osteogenic fronts of the condensations were maintained as proliferative osteoprogenitors (Twist1 and Runx2) to allow further growth. Surprisingly, the thin ventral mesenchyme between the opposing lateral plastron bone condensations was not undifferentiated but also showed osteogenic potential by expressing Runx2 by G15, and Runx2, Msx2, and Twist1 by G16 (Fig. 3 B and E–G, white arrows). The central area expressed the chondrogenic marker gene Sox9 at a minimal level at G15 but not at G16 (Fig. 3 A and C), and it did not express Collagen II (Fig. 3D). Preskeletal mesenchyme, before osteogenic or chondrogenic identity, expresses Prrx1 (42). Prrx1 regulates the formation of osteogenic and chondrogenic condensations, and it is found broadly expressed in the ventral mesenchyme in chicks at Hamburger Hamilton (HH)25 and in mice at embryonic day (E)12; but no expression of the osteogenic marker genes Runx2, Twist, or Msx2 are seen at this stage (32, 42–44). In turtles, at developmental stage G16, both the central ventral mesenchyme and the lateral regions of the ventral mesenchyme that correspond to plastron bone initiation sites expressed Prrx1 (Fig. 3I).
Fig. 3.
Osteogenic fate of plastron bone condensations and ventral mesenchyme in turtles. (A and B) At G15, the plastron bone condensations and ventral mesenchyme exhibited osteochondrogenic potential by being Sox9 positive (circled in A) with minimal Runx2 expression (B). (C and D) At G16, the chondrogenic potential of both the plastron bone condensations (orange circle in C) and the ventral mesenchyme had been suppressed. (E–H) By G16, an osteogenic condensation for each plastron bone had formed at the lateral edges of the developing plastron and the central area of the ventral mesenchyme had an osteogenic identity. Runx2 was expressed throughout the condensations (E). Its suppressor Twist1 was expressed by osteoprogenitor and preosteoblast cells in osteogenic fronts at the periphery of the condensations (F). Low level of Twist expression located between hyo- and hypoplastral condensations, and Msx2 was expressed by preosteoblasts in the center of the condensations (G). The ventral mesenchyme had osteogenic identity (white arrows in E–G). Osterix (Osx) was expressed in the core of the condensations, indicating osteogenic differentiation (H). A transient osteogenic condensation (black arrows in E–H) may represent the mesoplaston. (I) Prrx1 was expressed in preskeletal mesenchyme at G16 in both the osteogenic plastron condensates and ventral mesenchyme. Asterisks are adjacent to condensations. Yellow, hyoplastron; green, hypoplastron; blue, xiphiplastron. (Scale bar, ∼250 μm.)
Transient Mesoplaston.
The molecular analysis of osteogenic markers revealed that T. scripta embryos had a transient plastron bone condensation between the hyo- and hypoplastron condensations reminiscent of the mesoplastron (Fig. 3 E–H, black arrows). At G16, the potential mesoplastron primordium transiently expressed Msx2, Runx2, and minimal levels of Osx. A low level of Twist expression extended across the mesenchyme between the hyo- and hypoplaston. However, by G18, the mesoplastron condensation was no longer visible as a Runx2-positive area between hyo- and hypoplastron (Fig. S1). The μCT image of the PTA-stained G17 embryo did not reveal any condensation for mesoplastron, indicating that it does not secrete any significant amounts of extracellular matrix or that it has not reached a critical density that would render it detectable (Fig. 2A). The site of a transient mesoplaston was not aligned with the location of the scutes; rather it was positioned between pectoral and abdominal scutes (Fig. 4 C and E).
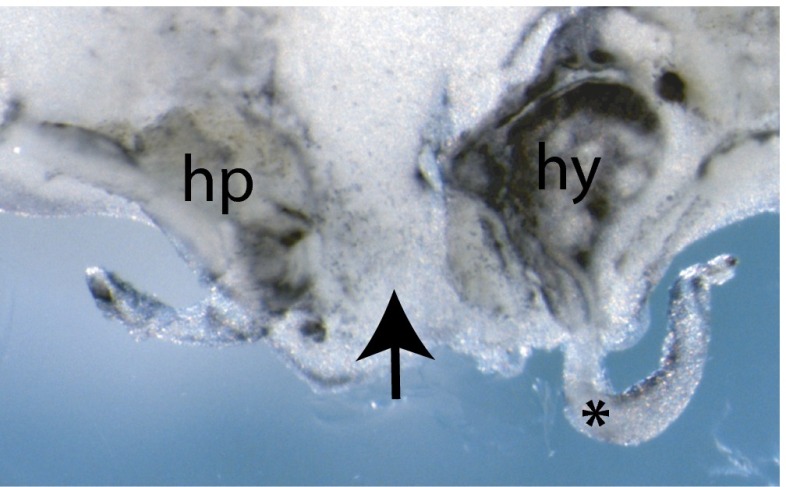
Fig. S1.
Transient mesoplastron condensation not visible from G18 onwards. Runx2 in situ hybridization of G18 plastron shows Runx2 expression at the osteogenic fronts of the hyoplastron (hy) and the hypoplastron (hp) as the bone plates are beginning to mature toward mineralization. There is no Runx2-positive mesoplastron between the hyo- and hypoplastron (black arrow). *, detached section of carapacial ridge.
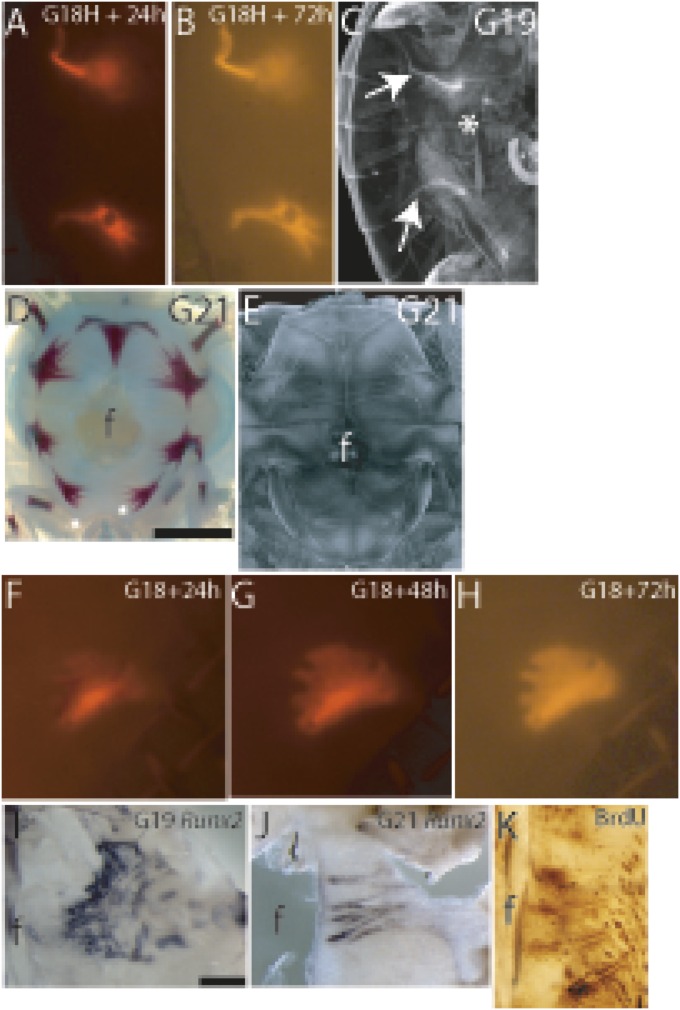
Fig. 4.
Development and patterning of bridge bones and bone spicules. (A and B) Incorporation of fluorescent calcein into cultured G18 plastron explants showed newly grown bone from the hyo- and hypoplastron bone plates (Upper and Lower, respectively) after a 24-h culture (A) and a 72-h culture (B). (C) µCT image of PTA-stained G19 embryo showed that bridge bones reached underneath the rib cage (white arrows). (D) G21 alizarin red staining and (E) μCT imaging of PTA-stained plastra demonstrated the extent of mineralized and unmineralized bone spicules. (F–H) G18 plastron explants cultured in vitro with fluorescent calcein. The hyoplastron explant after 24 h (F), 48 h (G), and 72 h (H) in culture showed the initiation of bone spicules at regular intervals toward the midline of the explant. The 72-h–cultured G18 hyoplastron resembled a G19 hyoplastron. (I) Bone spicules expressed Runx2. (J and K) Bone spicules grew by proliferative osteogenic fronts throughout their development; Runx2-positive osteogenic fronts at G21 (J) and BrdU incorporation into osteogenic fronts and the tips of the bone spicules at G21 (K). f, fontanelle.
Developmental Patterning of Plastron Bones.
To connect the dorsal and ventral exoskeletons, the hyo- and hypoplastron bones grow extensions from their lateral edges that reach underneath the rib cage in the carapace (Fig. 1, Movie S1). To follow bridge bone growth, we cultured G18 plastron explants in vitro with fluorescent calcein added to the culture medium to follow de novo bone deposition. After 24 h in culture, bridge bones had extended from the lateral edge of the plastron bone plates, and by 72 h they had grown to resemble the bridge bones seen in vivo at G19 (Fig. 4 A–C). In plastron explants, the carapace and ribs were not present to provide directional cue(s), yet the morphology resembled that of a bridge bone forming in vivo, indicating that the hyo- and hypoplastron bones rely on autonomous, intrinsic patterning information to grow bridge extensions.
By G21, the plastron bones have grown to cover a significant portion of the forming plastron. Each plastron bone plate was formed of a trabecular bone core, with extensive bone spicules radiating away from it and mineralizing (Figs. 1A and 4 D and E). MicroCT imaging showed that the bone spicules were much longer than alizarin red staining indicated; nonmineralized bone spicules traversed the midline of the plastron and crossed paths with their contralateral counterparts (hyo-, hypo, and xiphiplastron bones), and they also crossed paths ipsilaterally with ones from neighboring plastron bones (epi- and hyoplastron, hyo- and hypoplastron, and hypo- and xiphiplastron). The bone spicules organized themselves so as not to touch one another, and they often alternated with the ones from neighboring/opposing bone plates (Figs. 1A and 4 D and E).
Although the recruitment of the ossification pathways used in other intramembranous bones seems evident in the turtle plastron, there is no similar long and alternating bone spicule organization during avian or mammalian intramembranous ossification. To follow the development of bone spicules, we dissected G18 plastrons for organ culture supplemented with fluorescent calcein in the culture medium; after 24 h in culture, de novo bone deposition was seen mostly in the core of the plastron bone and no bone spicules were labeled. After a 48-h culture, short bone spicules were visible. By 72 h in culture, the mineralization was uniform throughout the bone plate but bone spicules had not grown longer. Short bone spicules radiated at even intervals along the medial edge of the cultured hyoplastron toward the midline (Fig. 4 F–H). The same pattern of plastron bone growth was seen in vivo at G19—short bone spicules radiating toward the midline from the medial edge of the uniformly mineralized bone plate (Fig. 4 I and J). Thus, G18 explants matured to G19 during the 3-d in vitro organ culture. The developing bone spicules were Runx2 positive, which was expressed in the osteogenic fronts surrounding the spicules. These osteogenic fronts surrounding the spicules were shown to be proliferative by BrdU incorporation (Fig. 4 I–K). Similarly to bridge bone extensions, bone spicules appeared to be part of the autonomous, intrinsic development and patterning of plastron bones driven by the osteogenic signaling.
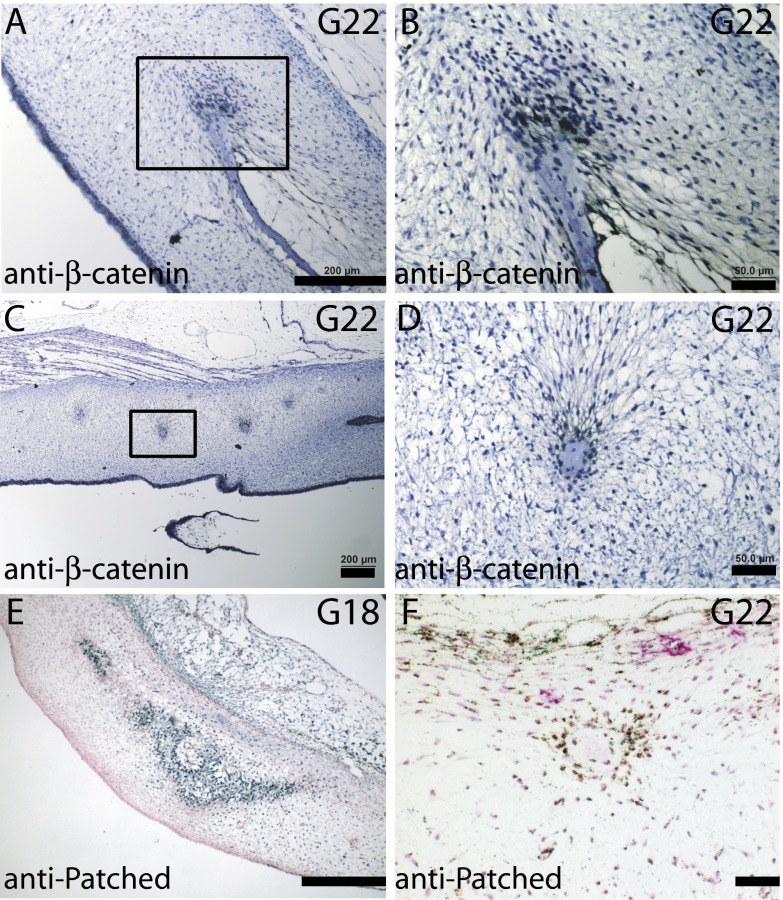
Sequential and overlapping Hedgehog and canonical Wnt signaling direct osteochondrogenic cells to choose an osteogenic path and prevent them from transdifferentiating into chondrocytes (15–17). Turtle preosteoblasts surround the plastron bone plate at G18, a stage that is before mineral deposition, and bone spicules at G22 showed nuclear localization of β-catenin (Fig. S2), indicating active canonical Wnt signaling. Patched protein was localized at the osteogenic fronts of plastron bones and bone spicules (Fig. S2), representing active Hedgehog signaling.
Fig. S2.
Osteogenic signaling in developing plastron bones and spicules. (A–D) Nuclear β-catenin staining of the cells at the proliferative osteogenic front of plastron bone (A and B) and surrounding bone spicules at G22 (C and D). (E) G18 transverse sections stained with anti-Patched antibody indicate Hedgehog signaling in the preosteoblastic cells in plastron bone, and (F) surrounding bone spicules at G22. [Scale bars, ∼200 μm (A, C, and E) and 50 μm (B, D, and F).]
Thus, the molecular regulation responsible for intramembranous bone formation and patterning in other vertebrates also drives plastron bone development in turtles. The critical difference appears to be the suppression of chondrogenesis in the ventral mesenchyme of the turtle, preventing abaxial cartilage structures from forming.
Discussion
All turtles have a unique body plan: an autapomorphic carapace on the dorsal side that contains dorsal ribs and carapacial bony plates, and a ventral shell of intramembranous bones that form a Chelonia-specific plastron. The dorsal and ventral shells develop as separate and different entities in turtles. The carapace contains endochondral axial skeletal elements, and during evolution its development has required multiple skeletal and muscular changes, including broadening of the ribs, shortening the trunk to nine dorsal vertebrates, dermal bone outgrowth from the perichondral collar, encapsulation of the scapula ventral to ribcage, and loss of intercostal muscles and reorganization of respiratory muscles (45, 46). Emergence of plastron bones in turtles has been thought to result from modifications of the pectoral girdle and gastralial bones (3, 4, 6, 8, 45). Meanwhile, the lack of cartilaginous sternum on the ventral side of turtles has not been previously considered from an evolutionary or molecular perspective.
All crown turtles discovered to date have had a full plastron. Several recent studies have shown turtles to be the sister group to the archosaurs (47–51), and a possible ancestral lineage has been proposed (45). The turtle plastron, its possible origin (52, 53) and homologies (6, 45) to skeletal elements found in other tetrapods have been studied and discussed for decades, yet this is the first study to our knowledge that has established a developmental timeline and location for plastron bone development from their early uncommitted mesenchymal condensations to the development and growth of bone extensions. This study also investigates the patterning information used in plastron bone growth.
The first sign of a turtle being a turtle during development is the formation of a signaling/organizing center called the carapacial ridge (CR) forming along the flank between fore- and hindlimbs at developmental stage G14 (26). By G16, the CR has grown to circle the dorsal carapacial disk that includes the ribs. The CR is essential for the turtle-specific rib phenotype—it attracts or patterns the spacing between ribs, and it is thought to be responsible for the lack of ventral ribs (25, 27, 33). This leads to the unusual situation in the turtle where the abaxial domain, the lateral plate mesenchyme, including elements that have invaded into it or migrated into it, is devoid of these endochondral skeletal elements. Instead, exoskeletal plastron bones have formed to provide support and protection to the ventral side of the body, and a muscular sheath encloses the lungs.
Here we have shown that plastron bone elements began to develop as individual mesenchymal condensations at developmental stage G15 at the periphery of the ventral mesenchyme (Fig. 3). At this stage, the first turtle-specific structure, the CR, has been established and rib growth will be limited to the primaxial domain of the embryo. Thus, just as the development of the carapace has begun, so has the development of the plastron. The mesenchymal condensations of intramembranous bones, such as those in the cranium, are Sox9-expressing osteochondrogenic condensations (10, 54). Soon after their formation, the chondrogenic potential is suppressed, and the condensed cells commit to osteogenesis. This appears to also be true in the turtle plastron: the early osteochondrogenic condensations at G15 suppressed their chondrogenic potential by G16 and committed to osteogenesis (Fig. 3). The plastron bone condensations contained the intrinsic patterning information necessary to grow into fully patterned plastron bones (Figs. 3, 4, and 5). Both the bridge bone extensions from hyo- and hypoplastron and the bone spicules grew in plastron explants in vitro to resemble those formed in vivo. Similarly, in the mouse, intramembranous bone plates have been shown to be autonomous from the surrounding mesenchyme during their growth and patterning, and no neighboring mesenchymal cells are recruited to the growing bone plates (55). Also, no chondrogenesis is allowed in the mesenchyme once osteogenic commitment is made (54).
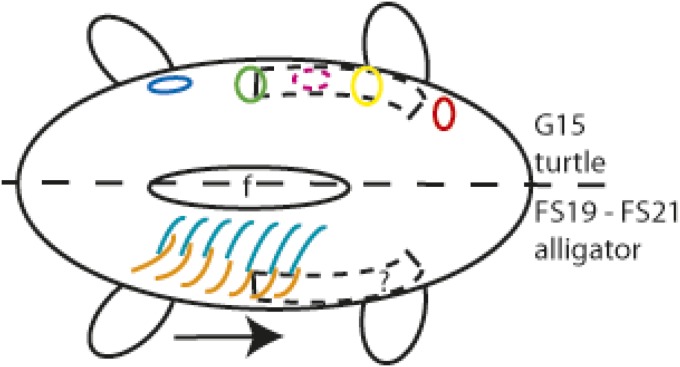
Fig. 5.
Hypothesis of sternum, plastron, and gastralia development. The turtle is depicted on the Top and the alligator at the Bottom of the schematic ventral side of an embryo. Anterior is to the Right. Chondrogenic sternal morphogenesis begins on lateral sides of the embryo extending from the posterior end of the forelimb posteriorly, based on chick and mouse studies (black dashed rectangle), but it does not form in turtles. This may be due to the osteogenic field of plastron bone condensations (circles) overlapping the sternal morphogenic area during the same developmental window. In alligators, it is not known when or where sternal morphogenesis begins. The gastralia begin to develop one to two developmental stages later than the plastron, and they develop in posterior-to-anterior sequence (black arrow); the lateral parts of the gastralial bones (orange) form first, and this is followed by the formation of the medial parts (blue). Gastralia lie posterior to the sternum. The osteogenic gastralia development is unlikely to coincide with chondrogenic sternal development. Diagram is not to scale. Circles: red, epiplastron; yellow, hyoplastron; magenta, transient mesoplastron; green, hypoplastron; blue, xiphiplastron; f, fontanelle.
In turtles, both the lateral regions of the ventral mesenchyme and the central region in between them were found to be osteogenic (Fig. 3). This osteogenic potential is maintained in the ventral mesenchyme later in development: mesenchymal cells isolated at G19 differentiated readily into osteoblasts in cell culture (53). At this stage bone spicules begin to form (Fig. 4) and osteogenic potential of the surrounding mesenchyme may support their growth.
The timing and location of the initial plastron bone condensations in the lateral regions of the ventral mesenchyme at G15 matched the developmental timing and location of sternal cartilage anlagen formation in avians (HH24 onwards) and mice (E12 onwards) (28, 30). Mutant mice that express ectopic Runx2 in the central ventral mesenchyme have no sternal cartilages at E15.5 and only malformed and unfused sternal cartilages at E18.5. This phenotype is caused by formation of interfering ectopic intramembranous bones in the ventral mesenchyme (32). In these mutant animals, the Prrx1 promoter was used to drive ectopic Runx2 expression in the central ventral mesenchyme starting at E9, which is before sternal morphogenesis at E12. The chondrogenic potential is not suppressed in these mutant mice and sternal cartilage anlagen are allowed to form.
Reptiles with intramembranous gastralia also have an endochondral sternum. Gastralia development begins by forming the posteriormost row of the paired lateral elements of gastralia first. This is followed by the development of the medial elements of the gastralia originating from the caudal end (34). As the first row of paired lateral parts of the gastralia form at developmental Ferguson stage (FS)19 and the first pair of medial parts appear in the caudalmost row at FS21 in alligator. Developmental stage FS19 in Alligator mississippiensis is approximately the same as G17 in T. scripta (56, 57). Thus, gastralial development begins at approximately one to two developmental stages later than plastron development. Also, the adult alligator gastralia remain posterior to sternum. Thus, it is possible that the development of gastralial elements does not overlap with the timing or location of sternal development in alligators (Fig. 5).
We suggest that in turtles, the suppression of chondrogenesis and the induction of osteogenesis in the ventral mesenchyme prohibit the formation of the sternum. In other words, turtles choose plastron bones.
Methods
Material.
T. scripta elegans eggs were purchased from the Kliebert Turtle and Alligator Farm. Animal work was performed in accordance with guidelines and approval from Finnish National Board of Animal Experimentation. Eggs were incubated in a humidified incubator at 30 °C. Embryos were staged according to Greenbaum (G) (57). T. scripta elegans’s total RNA was isolated from developmental stage G14 and G17 embryos and reverse transcribed into cDNAs, which were used as templates to clone T. scripta Osx, Prrx1, Runx2, and Sox9.
X-Ray μCT Imaging.
The 4% (wt/vol) PFA-fixed and 0.3% phosphotungstic acid (PTA)-stained samples were imaged with a custom-built phoenix X-ray Nanotom 180 NF (GE Measurement and Control Solutions) with X-ray tube voltage of 80 kV and tube current of 180 µA.
Organ Culture.
Dissected plastra were placed skin-side up on Nuclepore filters supported by grids in the Trowell type organ culture system (58). Details for all methods are available in SI Methods.
SI Methods
Cloning.
Primers for cloning Trachemys scripta Osx, Prrx1, Runx2, and Sox9 were designed against the sequences in T. scripta transcriptome (59). PCR products were purified and ligated into a vector, and the resulting plasmids were sequenced. Plasmids for T. scripta Msx2 and Twist have been published previously (60). DIG-labeled antisense cRNA probes were transcribed from linearized plasmid, and in situ hybridization on 4% (wt/vol) PFA-fixed whole mount samples was performed according to standard protocols.
X-Ray Microtomography Imaging.
The 4% (wt/vol) PFA-fixed embryos were dehydrated to 70% (vol/vol) ethanol, stained with 0.3% phosphotungstic acid (PTA) in 70% ethanol overnight to give contrast to soft tissues, and washed in 70% ethanol. The samples were imaged with a custom-built phoenix X-ray Nanotom 180 NF (GE Measurement and Control Solutions) with X-ray tube voltage of 80 kV and tube current 180 µA. The transmission images were acquired over a full 360° acquisition with 0.25° angular steps and formed from an average of 10 500-ms exposures. The effective pixel size of the transmission images was 9 µm. The 3D filtered back projection reconstructions were computed with software provided by the Nanotom manufacturer. Figures were done with either ImageJ (imagej.nih.gov/) or Amira (FEI Visualization Sciences Group and the Zuse Institute Berlin).
Organ Culture.
T. scripta embryos were rinsed and dissected in PBS with antifungal agents. Dissected plastra were cultured in medium [DMEM, 10% (wt/vol) FCS, GlutaMAX-1, 100 μg/mL ascorbic acid, 20 IU/mL penicillin–streptomycin, antifungal, and 90 μg/mL calcein (Sigma C-0875)] that was changed daily. Explants were cultured at 30 °C in 5% CO2. Cell proliferation was assessed by pulsed BrdU incorporation. Plastron explants were cultures as described (61). BrdU was added to the medium (3 μg/mL final concentration) of G22 plastron explants and cultured for 24 h before fixation. The streptavidin–biotin staining system (Invitrogen) was used to visualize BrdU according to the manufacturer’s instructions.
Immunohistochemical Staining.
The 4% (wt/vol) PFA-fixed paraffin sections were dewaxed, rehydrated, and permeabilized according to the manufacturer’s recommendations; the tissue sections were blocked, and incubated with anti-Patched (1:500, Santa Cruz Biotechnology) or anti–β-catenin (1:500, BD Biosciences) antibody overnight at 4 °C, then washed and incubated with secondary HRP-conjugated antibody (1:1,000, Invitrogen) overnight at 4 °C, developed using enzyme metallography EnzMet kit according to the the manufacturer’s protocol (Nanoprobes) and counterstained with nuclear fast red or hematoxylin.
Supplementary Material
Acknowledgments
We thank Agnès Viherä, Eija Koivunen, and Raija Savolainen for technical assistance and Profs. David Rice and Jukka Jernvall for comments on the manuscript. Funding was provided by the Academy of Finland (S.F.G.), the National Science Foundation (S.F.G. and J.C.-T.), Swarthmore College Faculty Research awards (to S.F.G.), Millersville University faculty research grants (to J.C.-T.), and the Pennsylvania State System of Higher Education (J.C.-T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600958113/-/DCSupplemental.
References
- 1.Schoch RR, Sues HD. A Middle Triassic stem-turtle and the evolution of the turtle body plan. Nature. 2015;523(7562):584–587. doi: 10.1038/nature14472. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Wu XC, Rieppel O, Wang LT, Zhao LJ. An ancestral turtle from the Late Triassic of southwestern China. Nature. 2008;456(7221):497–501. doi: 10.1038/nature07533. [DOI] [PubMed] [Google Scholar]
- 3.Cherepanov GO. The origin of the bony shell of turtles as a unique evolutionary model in reptiles. Russ J Herpetol. 1997;4:155–162. [Google Scholar]
- 4.Lyson TR, et al. Homology of the enigmatic nuchal bone reveals novel reorganization of the shoulder girdle in the evolution of the turtle shell. Evol Dev. 2013;15(5):317–325. doi: 10.1111/ede.12041. [DOI] [PubMed] [Google Scholar]
- 5.Claessens LPAM. Dinosaur gastralia; origin, morphology, and function. J Vertebr Paleontol. 2004;24(1):89–106. [Google Scholar]
- 6.Michael SYL. The homologies and early evolution of the shoulder girdle in turtles. Proc Biol Sci. 1996;263(1366):111–117. [Google Scholar]
- 7.Zangerl R. The Turtle Shell. Academic; London: 1969. p. 1969. [Google Scholar]
- 8.Owen R, Bell T. Monograph on the fossil reptilia of the London clay, and on the Bracklesham and other tertiary beds. Part I. Chelonia. Palaeontographical Society Monograph. 1849;2:1–76. [Google Scholar]
- 9.Rice DP, Rice R. Locate, condense, differentiate, grow and confront: Developmental mechanisms controlling intramembranous bone and suture formation and function. Front Oral Biol. 2008;12:22–40. doi: 10.1159/000115030. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama H, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102(41):14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng A, Genever PG. SOX9 determines RUNX2 transactivity by directing intracellular degradation. J Bone Miner Res. 2010;25(12):2680–2689. doi: 10.1002/jbmr.174. [DOI] [PubMed] [Google Scholar]
- 12.Rice DP, et al. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127(9):1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- 13.Bialek P, et al. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6(3):423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 15.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132(1):49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 18.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125(7):1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 20.Dodig M, et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999;209(2):298–307. doi: 10.1006/dbio.1999.9258. [DOI] [PubMed] [Google Scholar]
- 21.Hassan MQ, et al. Dlx3 transcriptional regulation of osteoblast differentiation: Temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene. Mol Cell Biol. 2004;24(20):9248–9261. doi: 10.1128/MCB.24.20.9248-9261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: Dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev. 2003;120(2):227–240. doi: 10.1016/s0925-4773(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 23.Yntema CL. Extirpation experiments on embryonic rudiments of the carapace of Chelydra serpentina. J Morphol. 1970;132(2):235–243. doi: 10.1002/jmor.1051320209. [DOI] [PubMed] [Google Scholar]
- 24.Rice R, Riccio P, Gilbert SF, Cebra-Thomas J. Emerging from the rib: Resolving the turtle controversies. J Exp Zoolog B Mol Dev Evol. 2015;324(3):208–220. doi: 10.1002/jez.b.22600. [DOI] [PubMed] [Google Scholar]
- 25.Burke AC. The development and evolution of the turtle body plan: Inferring intrinsic aspects of the evolutionary process from experimental embryology. Am Zool. 1991;31(4):616–627. [Google Scholar]
- 26.Burke AC. Development of the turtle carapace: Implications for the evolution of a novel bauplan. J Morphol. 1989;199(3):363–378. doi: 10.1002/jmor.1051990310. [DOI] [PubMed] [Google Scholar]
- 27.Nagashima H, et al. On the carapacial ridge in turtle embryos: Its developmental origin, function and the chelonian body plan. Development. 2007;134(12):2219–2226. doi: 10.1242/dev.002618. [DOI] [PubMed] [Google Scholar]
- 28.Chen JM. Studies on the morphogenesis of the mouse sternum. I. Normal embryonic development. J Anat. 1952;86(4):373–386. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JM. Studies on the morphogenesis of the mouse sternum. II. Experiments on the origin of the sternum and its capacity for self-differentiation in vitro. J Anat. 1952;86(4):387–401. [PMC free article] [PubMed] [Google Scholar]
- 30.Fell HB. The origin and developmental mechanics of the avian sternum. Philos Trans R Soc Lond B Biol Sci. 1939;229(563):407–463. [Google Scholar]
- 31.Kimura A, et al. Runx1 and Runx2 cooperate during sternal morphogenesis. Development. 2010;137(7):1159–1167. doi: 10.1242/dev.045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeno T, et al. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone. 2011;49(4):673–682. doi: 10.1016/j.bone.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert SF, Loredo GA, Brukman A, Burke AC. Morphogenesis of the turtle shell: The development of a novel structure in tetrapod evolution. Evol Dev. 2001;3(2):47–58. doi: 10.1046/j.1525-142x.2001.003002047.x. [DOI] [PubMed] [Google Scholar]
- 34.Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269(4):398–422. doi: 10.1002/jmor.10575. [DOI] [PubMed] [Google Scholar]
- 35.Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: Origin, evolution, and development. J Anat. 2009;214(4):441–464. doi: 10.1111/j.1469-7580.2008.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonçalves Vieira L, Quagliatto Santos AL, Campos Lima F, Souza Pinto JG. Ontogeny of the plastron of the giant Amazon River turtle, Podocnemis expanse (Schweigger, 1812) (Testudines, Podocnemididae) Zoolog Sci. 2009;26(7):491–495. doi: 10.2108/zsj.26.491. [DOI] [PubMed] [Google Scholar]
- 37.Werneburg I, Hugi J, Müller J, Sánchez-Villagra MR. Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dyn. 2009;238(11):2770–2786. doi: 10.1002/dvdy.22104. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Villagra MR, et al. Skeletal development in the Chinese soft-shelled turtle Pelodiscus sinensis (Testudines: Trionychidae) J Morphol. 2009;270(11):1381–1399. doi: 10.1002/jmor.10766. [DOI] [PubMed] [Google Scholar]
- 39.Sheil CA. Skeletal development of Macrochelys temminckii (Reptilia: Testudines: Chelydridae) J Morphol. 2005;263(1):71–106. doi: 10.1002/jmor.10290. [DOI] [PubMed] [Google Scholar]
- 40.Sheil CA. Osteology and skeletal development of Apalone spinifera (Reptilia: Testudines: Trionychidae) J Morphol. 2003;256(1):42–78. doi: 10.1002/jmor.10074. [DOI] [PubMed] [Google Scholar]
- 41.Metscher BD. MicroCT for developmental biology: A versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn. 2009;238(3):632–640. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- 42.Martin JF, Bradley A, Olson EN. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995;9(10):1237–1249. doi: 10.1101/gad.9.10.1237. [DOI] [PubMed] [Google Scholar]
- 43.Bell GW, Yatskievych TA, Antin PB. GEISHA, a whole-mount in situ hybridization gene expression screen in chicken embryos. Dev Dyn. 2004;229(3):677–687. doi: 10.1002/dvdy.10503. [DOI] [PubMed] [Google Scholar]
- 44.Darnell DK, et al. GEISHA: An in situ hybridization gene expression resource for the chicken embryo. Cytogenet Genome Res. 2007;117(1–4):30–35. doi: 10.1159/000103162. [DOI] [PubMed] [Google Scholar]
- 45.Lyson TR, Bever GS, Scheyer TM, Hsiang AY, Gauthier JA. Evolutionary origin of the turtle shell. Curr Biol. 2013;23(12):1113–1119. doi: 10.1016/j.cub.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Nagashima H, et al. Evolution of the turtle body plan by the folding and creation of new muscle connections. Science. 2009;325(5937):193–196. doi: 10.1126/science.1173826. [DOI] [PubMed] [Google Scholar]
- 47.Crawford NG, et al. More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett. 2012;8(5):783–786. doi: 10.1098/rsbl.2012.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field DJ, et al. Toward consilience in reptile phylogeny: miRNAs support an archosaur, not lepidosaur, affinity for turtles. Evol Dev. 2014;16(4):189–196. doi: 10.1111/ede.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fong JJ, Brown JM, Fujita MK, Boussau B. A phylogenomic approach to vertebrate phylogeny supports a turtle-archosaur affinity and a possible paraphyletic lissamphibia. PLoS One. 2012;7(11):e48990. doi: 10.1371/journal.pone.0048990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiari Y, Cahais V, Galtier N, Delsuc F. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria) BMC Biol. 2012;10:65. doi: 10.1186/1741-7007-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwabe N, et al. Sister group relationship of turtles to the bird-crocodilian clade revealed by nuclear DNA-coded proteins. Mol Biol Evol. 2005;22(4):810–813. doi: 10.1093/molbev/msi075. [DOI] [PubMed] [Google Scholar]
- 52.Cebra-Thomas JA, et al. Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev. 2007;9(3):267–277. doi: 10.1111/j.1525-142X.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 53.Cebra-Thomas JA, et al. Late-emigrating trunk neural crest cells in turtle embryos generate an osteogenic ectomesenchyme in the plastron. Dev Dyn. 2013;242(11):1223–1235. doi: 10.1002/dvdy.24018. [DOI] [PubMed] [Google Scholar]
- 54.Aberg T, Rice R, Rice D, Thesleff I, Waltimo-Sirén J. Chondrogenic potential of mouse calvarial mesenchyme. J Histochem Cytochem. 2005;53(5):653–663. doi: 10.1369/jhc.4A6518.2005. [DOI] [PubMed] [Google Scholar]
- 55.Lana-Elola E, Rice R, Grigoriadis AE, Rice DP. Cell fate specification during calvarial bone and suture development. Dev Biol. 2007;311(2):335–346. doi: 10.1016/j.ydbio.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson MJ. The reproductive biology and embryology of crocodilians. In: Gans C, Billet F, Maderson PFA, editors. Biology of the Reptilia. Wiley; New York: 1985. pp. 329–491. [Google Scholar]
- 57.Greenbaum E. A standardized series of embryonic stages for the emydid turtle Trachemys scripta. Can J Zool. 2002;80(8):1350–1370. [Google Scholar]
- 58.Thesleff I, Sahlberg C. Organ culture in the analysis of tissue interactions. Methods Mol Biol. 2008;461:23–30. doi: 10.1007/978-1-60327-483-8_3. [DOI] [PubMed] [Google Scholar]
- 59.Kaplinsky NJ, et al. The embryonic transcriptome of the red-eared slider turtle (Trachemys scripta) PLoS One. 2013;8(6):e66357. doi: 10.1371/journal.pone.0066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moustakas JE. Development of the carapacial ridge: Implications for the evolution of genetic networks in turtle shell development. Evol Dev. 2008;10(1):29–36. doi: 10.1111/j.1525-142X.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 61.Cebra-Thomas J, et al. How the turtle forms its shell: A paracrine hypothesis of carapace formation. J Exp Zoolog B Mol Dev Evol. 2005;304(6):558–569. doi: 10.1002/jez.b.21059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.