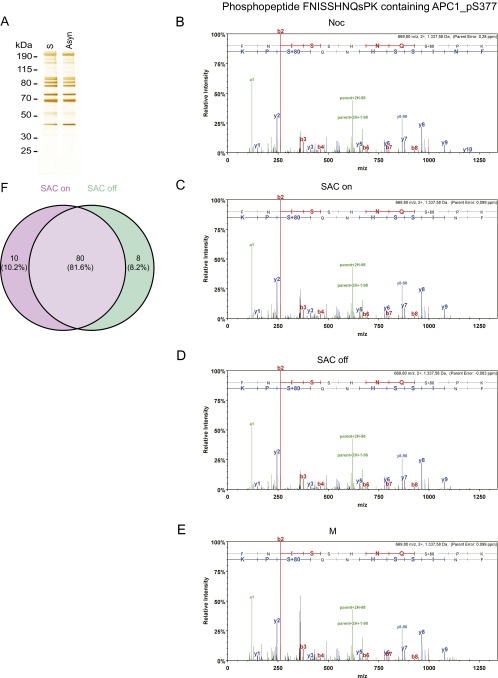
Fig. S1.
Mass spectrometry studies on various forms of APC/C immunopurified from HeLa cells. (A) Silver stain of APC/C immunoprecipitated using APC3 antibody beads from asynchronous cells and double-thymidine arrest–release synchronized cells (S-phase). (B–E) Tandem mass spectra of the phospho-S377 containing phospho-peptide FNISSHNQsPK in APC1 derived from (B) nocodazole-arrested HeLa cells (Noc), (C) Taxol + MG132-arrested cells (SAC on), (D) Taxol + MG132 + hesperadin-arrested cells (SAC off), and (E) cells treated with double-thymidine arrest–release procedure (M). Spectra of the phospho-peptides were acquired by higher energy collisional dissociation of the (M + 2H)2 + precursor, m/z 669.80 (B–E). Fragment ions in the spectra represent single-event preferential cleavage of the peptide bonds resulting in the sequence information recorded simultaneously from both the N and C termini (b- and y-ions, respectively) of the peptide. (F) Venn diagram showing that phospho-sites mapped on SAC on and SAC off samples largely overlap. The SAC on samples contained 10 phospho-peptides that were not found in the SAC off samples, although the corresponding nonphosphorylated peptides were recovered in the SAC off samples. Conversely, the SAC off samples contained seven phospho-peptides that were not found in the SAC on samples, although the corresponding nonphosphorylated peptides were recovered in the SAC on samples. We also identified one phospho-peptide in the SAC off samples for which the corresponding nonphosphorylated peptides were not found in SAC on samples. All phospho-peptides are listed in Dataset S3.