Figure 9. GEAT361 lineage thermostability and footprints shift from Imm 2 to Imm 5.
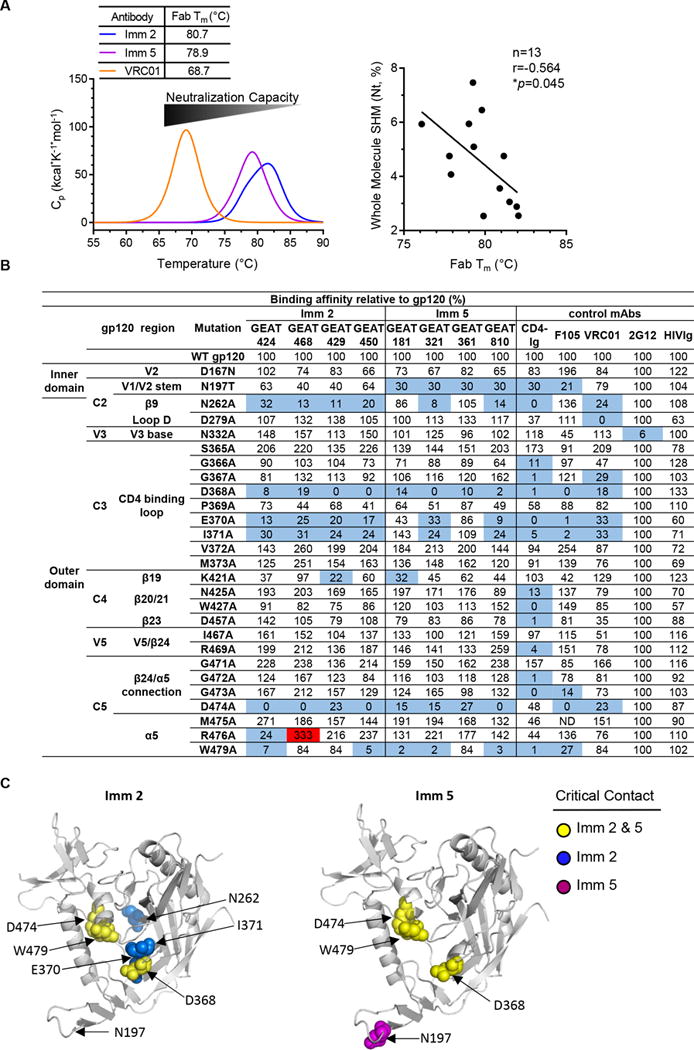
(A) The antibody affinity maturation is associated with the change of thermal stabilities of the free antibody molecule. Left, the average DSC melting profiles of the Fab portion of the mAbs (Fab Tm) from the Imm 2 (blue) and Imm 5 (purple) time points. Note that from Imm 2 to Imm 5 time point, the average Fab Tm decreases, conversely with the increasing trend of antibody affinity for gp120 and virus neutralization capacity. The bNAb VRC01 (orange) has an even lower Fab Tm. Right, correlation between the whole molecule SHM (Nt) and the Fab Tm of the cloned mAbs (non-parametric Spearman test). (B) Representative clones from the GEAT361 linage and selected human antibodies as references were tested to bind a panel of JRCSF Env mutants containing single Ala point mutations in gp120. Both 2G12 and HIVIg served as controls. The effect of Ala mutation on antibody binding is shown relative to wild-type (WT) gp120. A three-fold reduction by mutation was highlighted in blue and a three-fold increase highlighted in red. (C) gp120 core surface (PDB: 2NY3) used to highlight the recognition sites on gp120 by selected GEAT361 clonal variants inferred by Ala scanning. Left, footprints of Imm 2 mAb variants of lower affinity maturation level. Right, footprints of the variants with the highest degree of affinity maturation, GEAT181 and 361, isolated from Imm 5 time point. gp120 backbone is in grey and residues affecting antibody binding for both Imm 2 and Imm 5 variants highlighted in yellow, while mutations on residues only affecting binding at Imm 2 or Imm 5 are highlighted in blue and purple, respectively.
