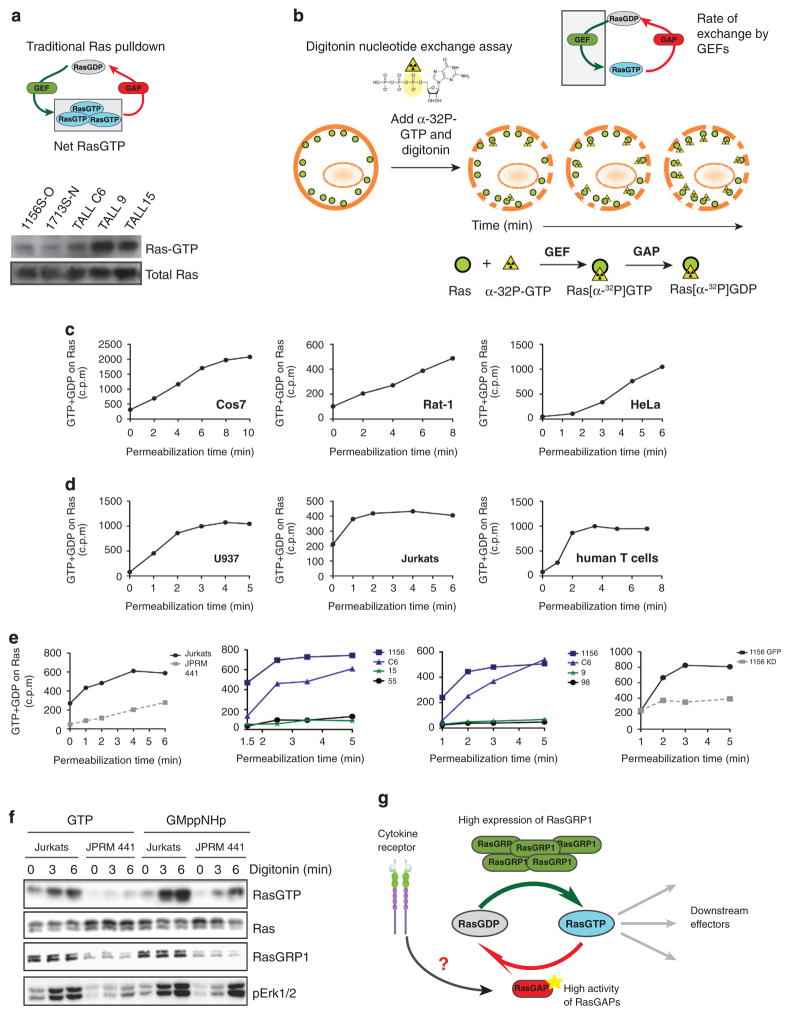
Figure 6.
Overexpression of RasGRP1 leads to high rate flux through RasGDP/RasGTP cycle. (a) Scheme of traditional Ras pulldown assay that measures net RasGTP levels and western blot analysis of RasGTP after Ras pulldown in RasGRP1 (1156S-O, 1713S-N and T-ALL C6) and KRasG12D (T-ALL 9, TALL15) T-ALL cell lines. (b) Scheme of digitonin exchange assay that measures loading of GTP into Ras. In short, cells are permeabilized with digitonin and loaded with radioactively labeled GTP nucleotide. GTP is labeled at the α position; therefore, after GAP-mediated hydrolysis, the radioactive label is not released and Ras-bound radioactivity accumulates over time. Ras is then immunoprecipitated and GTP loading quantified by scintillation counting. (c and d) Graphs depict rate of accumulation of radiolabeled guanine nucleotide in counts per minute that was loaded onto Ras in the basal state over time as measured with the digitonin exchange assay. Assays were carried out in a panel of cell lines that either do not express RasGRP proteins or have low expression of RasGRP1 and 3 (Cos7, Rat1, HeLa) (c) and compared with cell lines that express high levels of RasGRP1 or 4 (Jurkats, human T cells, myeloid U937) (d). (e) Accumulation of radiolabeled guanine nucleotide in counts per minute in resting cells on Ras as measured with the digitonin exchange assay in a panel of T-ALL cell lines. Left panel compares human T-ALL line, Jurkat and its derivative JPRM441, which express low levels of RasGRP1; two middle panels compare RasGRP1 T-ALL cell lines (1156, C6) with KRasG12D (T-ALL 15 and 9). Two T-ALL cell lines without known mutations in the Ras pathway (T-ALL 55 and 98) are shown as controls. Far right panel compares 1156S-O GFP cell line with 1156S-O cell line with stable shRNA-mediated knockdown of RasGRP1 (1156 KD).5 Panels c–e are representative examples at least two independent experiments. (f) Biochemical RasGTP determination in Jurkat T-ALL cell line and JPRM441 derivative expressing low levels of RasGRP1 treated with digitonin and loaded with either GTP or its non-hydrolyzable GTP analog GMppNHp for the indicated periods of time. (g) A proposed model elucidating a potential tumor suppressor role of RasGAPs in RasGRP1 T-ALL cells. Dysregulated RasGRP1 uses basal DAG to anchor to the membrane to perform exchange of GDP to GTP on Ras. High loading of GTP is counterbalanced by increased activity and/or expression of RasGAPs to hydrolyze GTP back to GDP and maintain low RasGTP levels. Cytokine receptor activation disrupts this cycle by acting on RasGAPs to decrease their activity resulting in the accumulation of RasGTP in the cell.