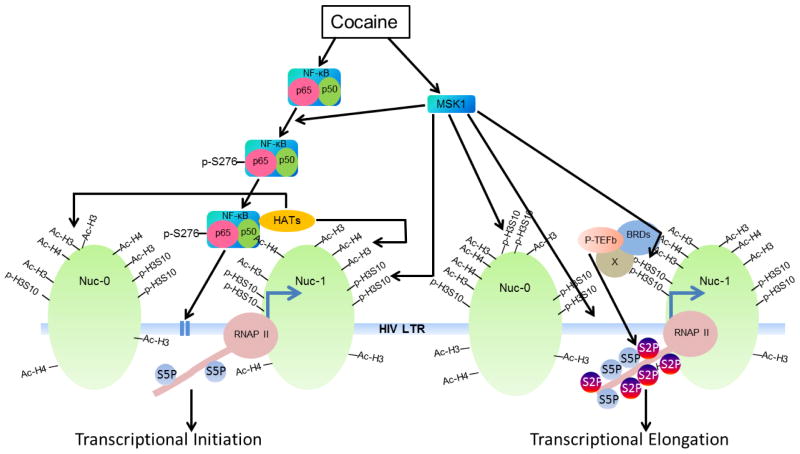
Figure 2. Schematic diagram showing cocaine accelerates HIV transcription by promoting both initiation and elongation phases of HIV transcription.
Cocaine treatment results in the activation and recruitment of NF-κB and MSK1 at HIV LTR. MSK1 subsequently catalyze the phosphorylation of NF-κB at its serine residue 276. Phosphorylation of NF-κB at 276th serine residue enhances the functional capability of NF-κB by augmenting the interaction of NF-κB with HATs. HATs such as p300 in turn catalyze the acetylation of core histones (mainly H3 and H4) at HIV LTR and facilitate the establishment of transcriptionally active chromatin structures, which boost the access of transcription machinery to HIV LTR and thus facilitate the initiation phase of HIV transcription. MSK1 in addition to phosphorylating NF-κB catalyzes the phosphorylation of histone H3 at serine 10 (H3S10). Phosphorylated histone H3 at 10th residue contributes to the establishment of transcriptionally active chromatin structure at HIV LTR. Moreover, phosphorylated H3S10 also facilitates the recruitment of P-TEFb at HIV LTR through a mechanism that is not yet fully defined; usually with the help of BRD proteins which bind specifically to acetylated histones through their bromo domain. P-TEFb, an established elongation factor, subsequently catalyzes the phosphorylation of several proteins, including RNA Polymerase II (RNAP II) and negative elongation factors. These modifications enhance the processivity of RNAP II and nullify the impact of negative elongation factors, and thus promote the elongation phase of HIV transcription.