Figure 6. RIBEYE KO severely impairs neurotransmitter release at bipolar cell/AII amacrine cell synapses as monitored by paired recordings in acute retina slices.
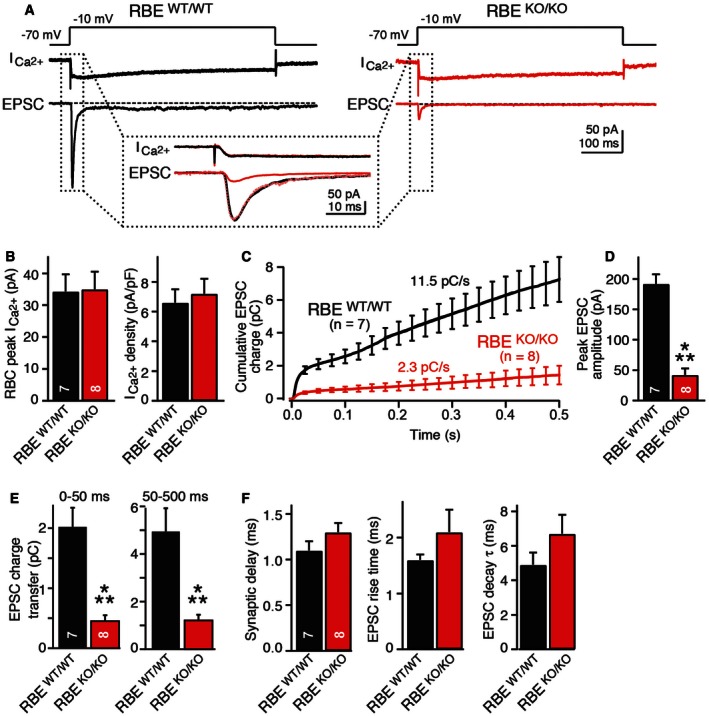
- Experimental design and representative traces for analysis of synaptic transmission in retinas from wild‐type (black) and RIBEYE KO mice (red). Presynaptic bipolar neurons were depolarized in voltage‐clamp mode from −70 to −10 mV for 0.5 s as indicated on top to open Ca2+ channels and trigger release. Presynaptic Ca2+‐currents and postsynaptic EPSCs were measured simultaneously as indicated in the traces; dotted box displays an expansion of the initial Ca2+‐currents and EPSCs.
- RIBEYE KO does not alter presynaptic Ca2+‐currents. Summary graphs show average peak Ca2+‐currents and Ca2+‐current.
- RIBEYE KO severely impairs EPSCs triggered by presynaptic depolarization. Plot shows integrated EPSC charge as a function of time; both initial and sustained neurotransmitter releases are impaired.
- RIBEYE KO strongly reduces initial synchronous release induced by presynaptic depolarization as indicated by the summary graph of the peak EPSC amplitude.
- RIBEYE KO suppresses both the initial synchronous and the sustained phase of release induced by presynaptic depolarization. Summary graphs display integrated EPSC charges for the initial phase (0–50 ms) and the sustained phase (50–500 ms).
- RIBEYE KO does not alter the kinetics of EPSCs. Summary graphs depict the mean synaptic delays (left), rise times (center), and decay time constants (right) of EPSCs.
