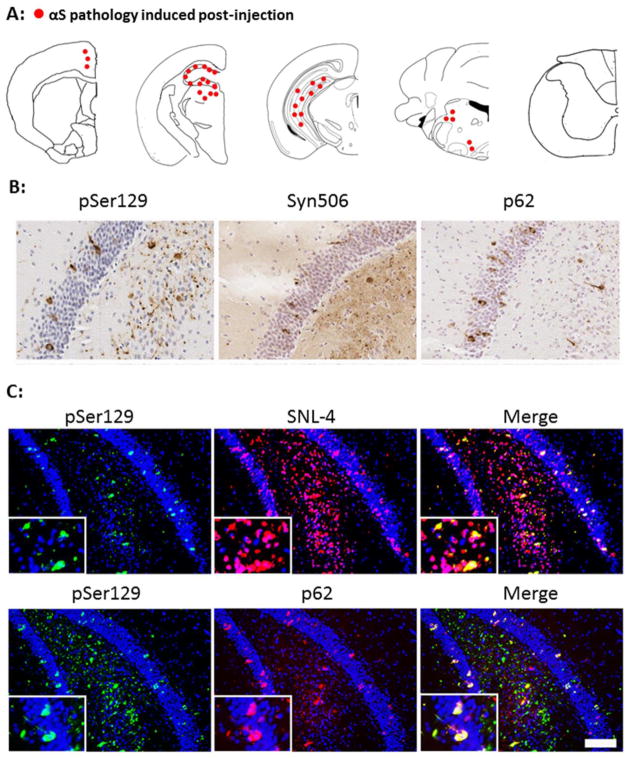
Fig 3. Characterization and distribution of αS inclusion pathology at 4 months post intrahippocampal injection of 21–140 hfib αS in M47 Tg mice.
(A) Diagram shows rostral/caudal distribution of αS inclusion pathology in M47 Tg mice. Tissue sections were stained with antibodies pSer129/81A and Syn506 to detect αS inclusion pathology. Intrahippocampal injections resulted in the induction of αS pathology at the site of injection with some additional pathology in the midbrain and brainstem. Similar density and distribution of αS pathology was seen bilaterally. (B) Brain tissue sections with representative regions of the hippocampus in injected M47 Tg mice show rounded, perinuclear inclusions and neuritic pathology stained with pSer129/81A. Inclusions were also recognized by Syn506, a mouse monoclonal antibody that conformationally recognizes αS inclusions, and p62, a rabbit polyclonal antibody that non-specifically recognizes protein aggregates. (C) Double immunofluorescence analysis of the hippocampal region stained for pSer129/81A (green) and SNL-4 (red; upper panels) or p62 (red; lower panels) shows that most hyperphosphorylated αS inclusions are SNL-4+ and that a large portion are also p62+ in M47 Tg mice injected with 21–140 hfib αS. There is also significant p62 immunoreactivity that does not co-localize with pSer129 indicating the formation of more than one type of protein aggregate. Tissue sections were counterstained with hematoxylin (B) and DAPI (C). Scale bar = 50 μm (B), and 100 μm and 25 μm (C; insets).