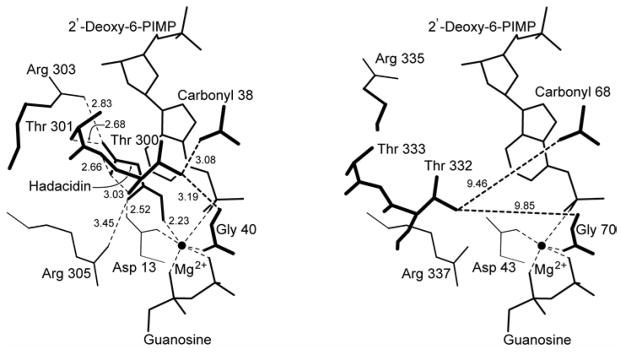
Figure 4. Schematic of the aspartyl pocket of mouse muscle adenylosuccinate synthetase.
Distances (in Å) in the 2′-deoxy-6-PIMP•GDP•hadacidin (fully ligated) complex and the 2′-deoxy-6-PIMP•GDP (partially ligated) complex are in the left and right panels, respectively. Distances from Thr300 OG1 to carbonyl 38 and amide 40 (corresponding to distances from Thr332 OG1 to carbonyl 68 and amide 70 in the mouse enzyme) provide a measure of the movement of the Asp Loop (See Figure 1 for definition) relative to the active site.