Table 1. Optimization of Ni-NIXANTPHOS catalyzed DCCP of 1a with 2a a .
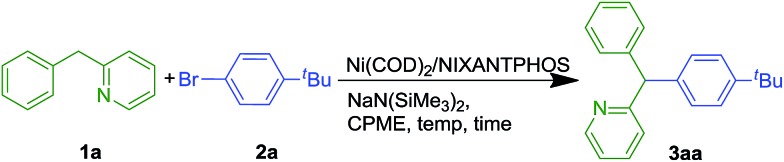
| ||||||
| Entry | 1a : 2a : base | Ni/L (mol%) | T (°C) | t (h) | Concn | Yield b (%) |
| 1 | 1.2 : 1 : 3 | 10/10 | 110 | 16 | 0.1 | 78 |
| 2 | 1.2 : 1 : 3 | 10/15 | 110 | 16 | 0.1 | 71 |
| 3 | 1.2 : 1 : 3 | 10/20 | 110 | 16 | 0.1 | 62 |
| 4 | 1.2 : 1 : 2 | 10/10 | 110 | 16 | 0.1 | 83 |
| 5 | 1.2 : 1 : 2 | 10/10 | 110 | 16 | 0.05 | 85 |
| 6 | 1 : 1.5 : 2 | 10/10 | 110 | 16 | 0.05 | 93 |
| 7 | 1 : 1.5 : 2 | 10/10 | 110 | 8 | 0.05 | 77 |
| 8 | 1 : 1.5 : 2 | 10/10 | 110 | 24 | 0.05 | 81 |
| 9 | 1 : 1.5 : 2 | 10/10 | 50 | 16 | 0.05 | 99 |
| 10 | 1 : 1.5 : 2 | 10/10 | rt | 16 | 0.05 | 99 |
| 11 c | 1 : 1.5 : 2 | 2.5/2.5 | rt | 16 | 0.05 | 99(99) |
| 12 | 1 : 1.5 : 2 | 1/1 | rt | 16 | 0.05 | 72 |
aReactions conducted on a 0.10 mmol scale.
bYield determined by 1H NMR spectroscopy of the crude reaction mixtures.
cIsolated yield after chromatographic purification.
