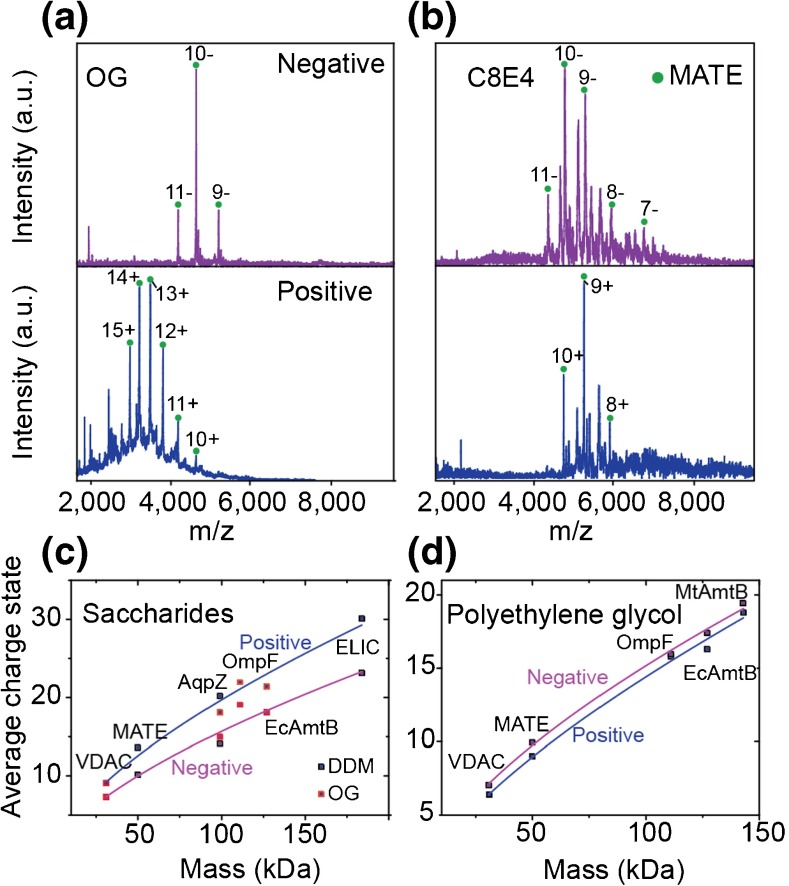
Figure 2.
The effect of negative polarity on charge state in the presence of detergent. Mass spectra of MATE solubilized in OG (a) and C8E4 (b), in negative polarity (top, purple), and positive polarity (bottom, blue). A charge reduction was observed in the case of OG (left panel), whereas no charge reduction was observed for C8E4 (right panel). For the proteins investigated (Table S1) in saccharide detergents (c), a charge reduction is observed between positive (blue) and negative polarity (purple). The data are fitted to a power low (z ave = aM b), where z ave is the average charge and M is mass in kDa, with a and b values of 0.99 and 0.65 for positive polarity and 0.78 and 0.65 for negative polarity. The R 2 of the fits were 0.97 and 0.95 for positive and negative ion modes respectively. (d) The dependence of average charge state on mass in polyethylene glycol detergents (C8E4) in both positive and negative polarity. The data were fitted with a power law with a and b values of 0.80 and 0.64 for negative mode and 0.61 and 0.69 for positive mode. The R 2 of both fits was 0.99