Figure 1.
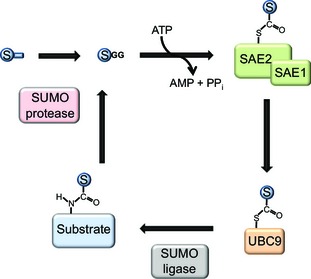
The SUMOylation cycle. First, the SUMO precursor protein is cleaved to its mature form by SUMO proteases, exposing a C‐terminal di‐glycine motif. In an ATP‐dependent reaction, the C‐terminus of SUMO is attached via a thioester bond to the SUMO activating enzyme, consisting of the two subunits SAE1 and SAE2. It is then further transferred to an internal cysteine of the SUMO conjugating enzyme (UBC9). In the third step, SUMO is attached to a lysine residue of a target protein, a process that is facilitated by the presence of a SUMO ligase. Finally, SUMO proteases cleave SUMO from its substrate, resulting in free SUMO that re‐enters the SUMOylation cycle.
