Figure 4.
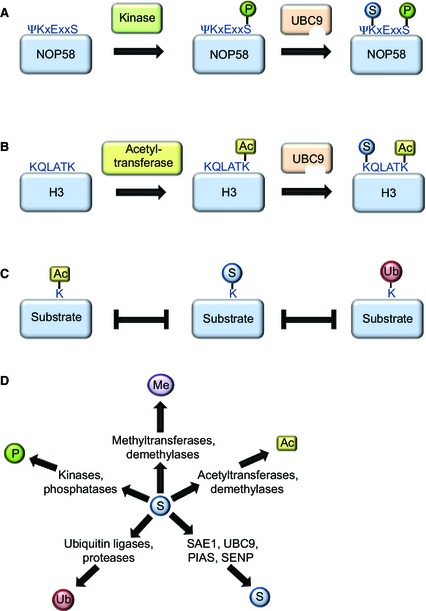
Cross‐talk between post‐translational modifications as identified via mass spectrometry. (A) Several SUMO target proteins contain a so‐called phosphorylation‐dependent SUMO motif, in which the modified lysine residue is followed by a phosphorylated serine, usually five amino acids further downstream. A serine residue situated in a phosphorylation‐dependent SUMO motif of the nuclear protein NOP58 is phosphorylated via casein kinase 2 (CK2), promoting UBC9 binding and subsequent SUMOylation of the indicated lysine residue. (B) Similar to phosphorylation, acetylation via specific acetyl transferases may induce SUMOylation of a protein, as described for histone H3. (C) Many lysine residues that were found as SUMO acceptor sites have also been shown to be ubiquitylated or acetylated, suggesting extensive competition between these modifiers. (D) Dozens of enzymes regulating post‐translational protein modifications have been identified as SUMO target proteins in proteomic screens, including SUMO pathway components (S), or enzymes regulating other post‐translational modifications, such as phosphorylation (P), ubiquitylation (Ub), methylation (Me) or acetylation (Ac).
