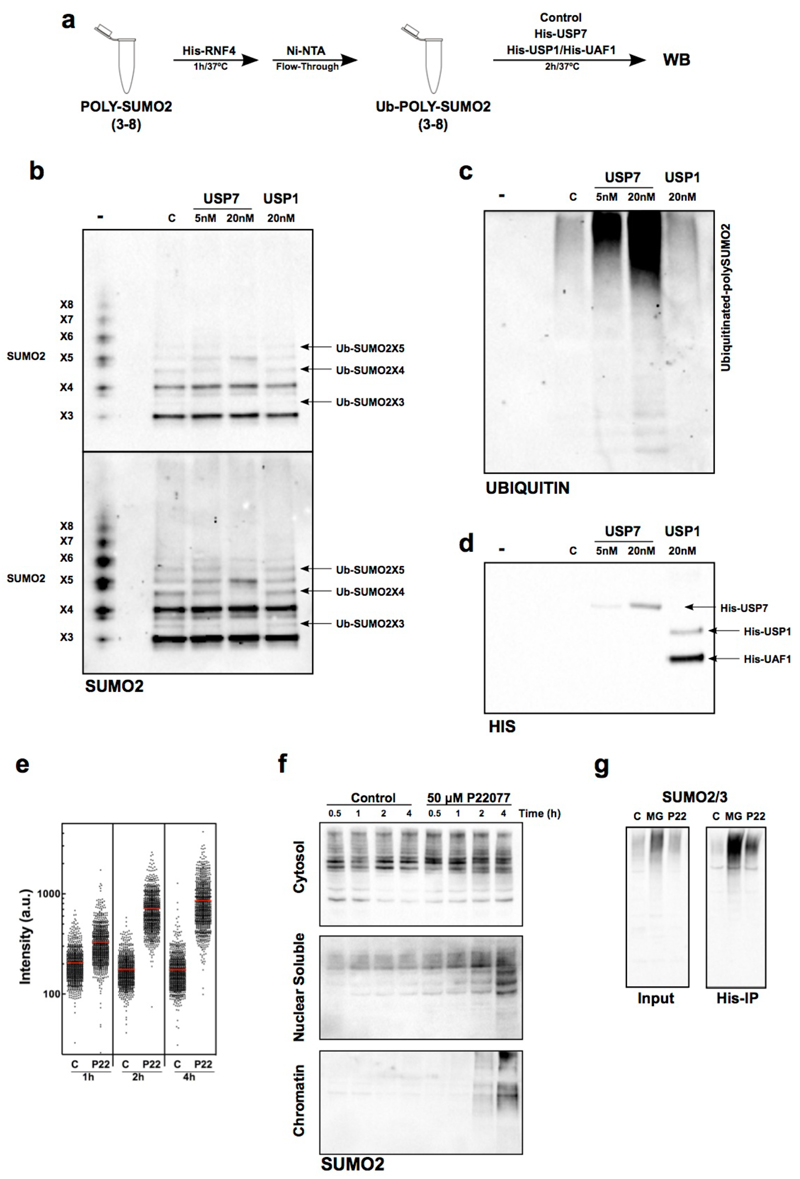
FIG. 4. USP7 DEUBIQUITINATES SUMO2 IN VITRO AND IN VIVO.
(a) Scheme of the biochemical assay used to test SUMO2 deubiquitination in vitro.
(b-d) In vitro ubiquitinated poly-SUMO2 chains were incubated with His-USP7, His-USP1/His-UAF1 or no enzyme (C). Non-ubiquitinated poly-SUMO2 chains are shown as reference (-). Reaction products were analyzed by WB with antibodies against SUMO2/3 (b), ubiquitin (c) or His (d). One out of two representative experiments is shown. Note that the loss of ubiquitinated poly-SUMO2 bands occurs concomitantly to the increase in signal in the bands corresponding to unmodified SUMO2 polymers.
(e) The intensity of chromatin-bound SUMO2/3 per individual nucleus upon USP7 inhibition as quantified by HTM. U2OS cells treated with 50 µM P22077 or DMSO (C) for the indicated times were fixed and stained for SUMO2/3 after pre-extraction of the nuclear-soluble proteins. DAPI was used to stain nuclei.
(f) Cytosolic, nuclear-soluble and chromatin fractions were purified from HCT116 cells treated with 50 µM P22077 for the indicated times. The presence of SUMOylated proteins was measured by WB with antibodies against SUMO2/3. The experiment was repeated twice and one representative image is shown.
(g) 293T cells were transiently transfected with a plasmid encoding His-ubiquitin and treated with 5 µM MG132 (MG), 50 µM P22077 (P22) or DMSO (C) for 4 h. Nuclear extracts were obtained and ubiquitin containing proteins were purified using a Ni-NTA resin. The presence of SUMOylated proteins in the input or the His-IP was followed by WB with specific antibodies for SUMO2/3.