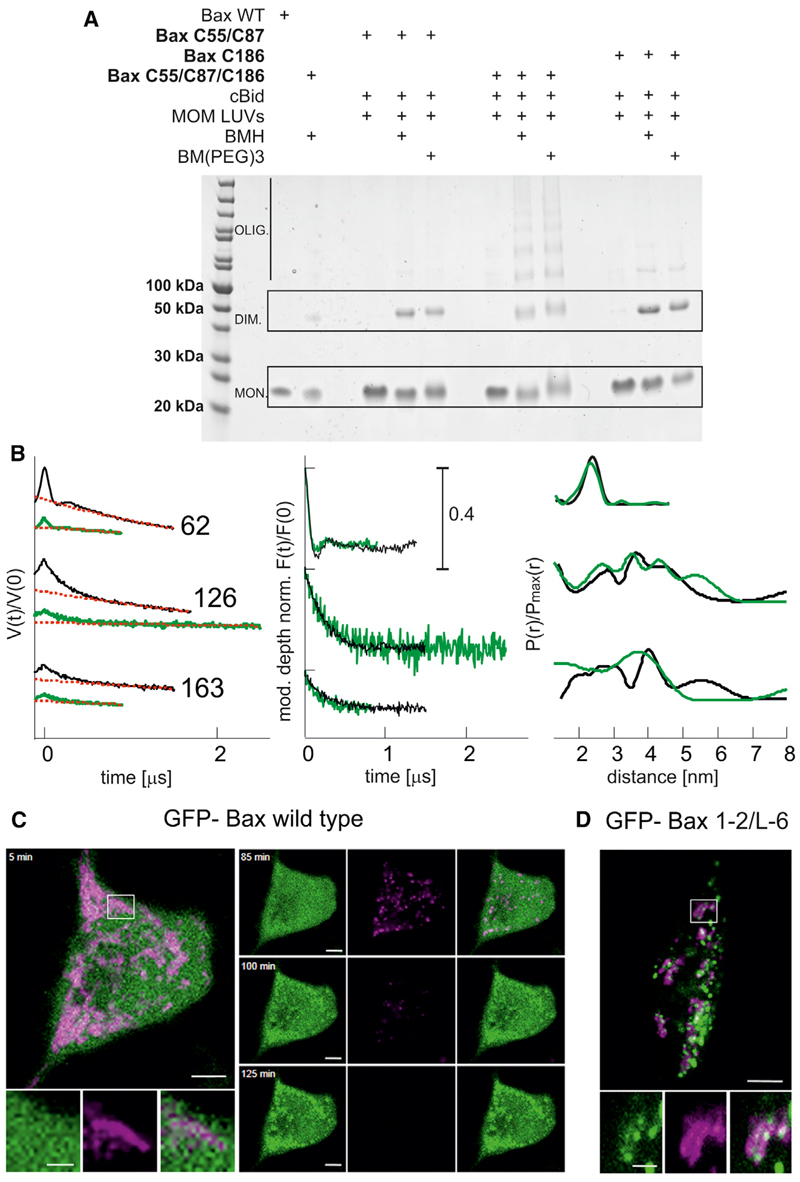
Figure 5. Validation of the Proposed Model.
(A) Helix 9 is involved in interdimer interactions, as proven by crosslinking. Three different Bax mutants are used to selectively connect the proposed intradimer interface (Bax C55, C87) and the proposed interdimer interface (Bax C186) or both interfaces (Bax C55, C87, C186) within membrane-embedded Bax oligomers. The bismaleimide crosslinkers BMH (1.3 nm) and BM (PEG)3 (1.8 nm) were used.
(B) Bax adopts the same conformation in liposomes and mitochondria. Left: experimental Q-band DEER traces V(t)/V(0) with background function (dotted red) performed with DeerAnalysis2013 (Jeschke et al., 2006). Color code: black, data in liposomes; green, data in isolated mitochondria. Middle: modulation depth normalized background corrected traces (F(t)/F(0)). Right: comparison of the obtained distance distributions.
(C) Bax-GFP is soluble in healthy HeLa cells and translocates to mitochondria concomitant with potential loss upon apoptosis induction (control experiment).
(D) Crosslinked GFP-Bax 1-2/L6 is trapped in a globular protein fold and is constitutively bound to the mitochondrial membrane without inducing permeabilization. Color code: GFP-Bax, green; membrane potential sensitive dye TMRE, magenta. Scale bar represents 5 μm and 2 μm for zoomed images. At t = 0, 1 μM staurosporine was added to the cells.