Fig. 2.
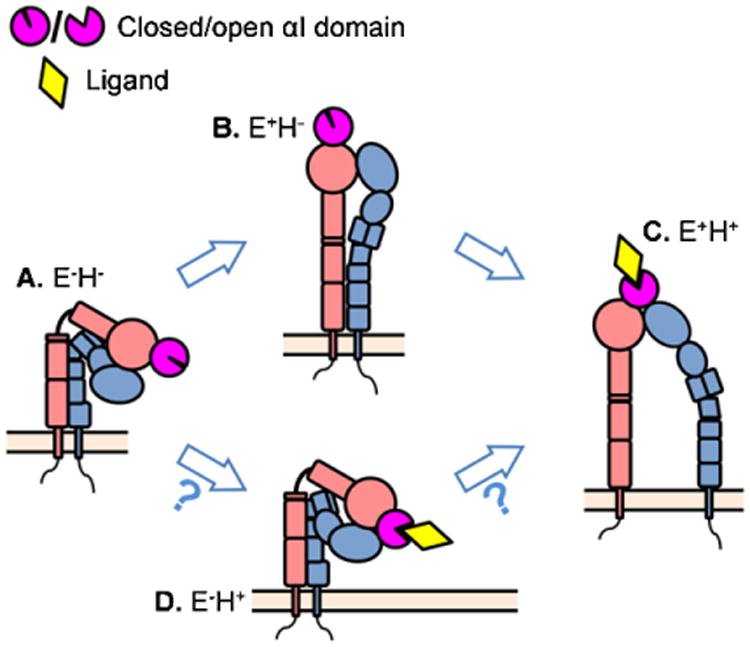
The existing conformations of the β2 integrins and the proposed consequences of conformational changes. (A) The resting state of β2 integrin has bent ectodomain with closed headpiece. (B) The resting β2 integrin can extend its ectodomain. This conformation may have intermediate affinity to the ligand and mediate leukocyte slow rolling. (C) Further conformational changes can induce headpiece-opening and acquire fully activated β2 integrin. This conformation has high affinity to the ligands and is thought to support arrest and leukocyte adhesion. (D) The structure of bent ectodomain with open headpiece was also crystallized in β2 integrin.
