Fig. 2.
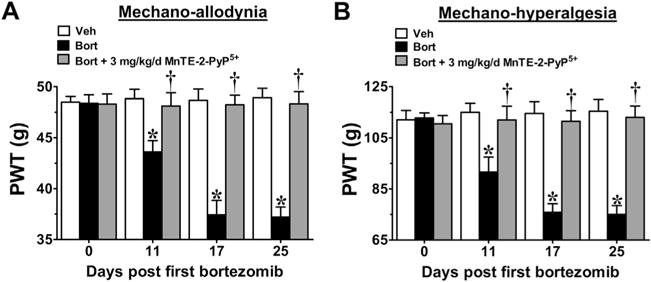
Mn(III) 5,10,15,20-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+) prevents the development of bortezomib (Bort)-induced neuropathic pain. Compared with baseline [day (D)0], administration of bortezomib (black bars), but not its vehicle (Veh) (open bars), led to the development of mechano-allodynia (A) and mechano-hyperalgesia (B). Prophylactic daily injections (D0–17) of MnTE-2-PyP5+ (3 mg/kg/d; gray bars) blocked the development of both bortezomib-induced mechano-allodynia (A) and mechano-hyperalgesia (B). Hypersensitivity did not appear after drug termination up until the end of testing on D25 (A, B). Results are expressed as mean ± SD, n = 5–6, and analyzed by two-way analysis of variance with Bonferroni’s post-hoc comparisons. *P < .001 (tday vs tday 0); †P < .001 (Bort + MnTE-2-PyP5+ vs Bort). PWT = paw withdrawal threshold.
