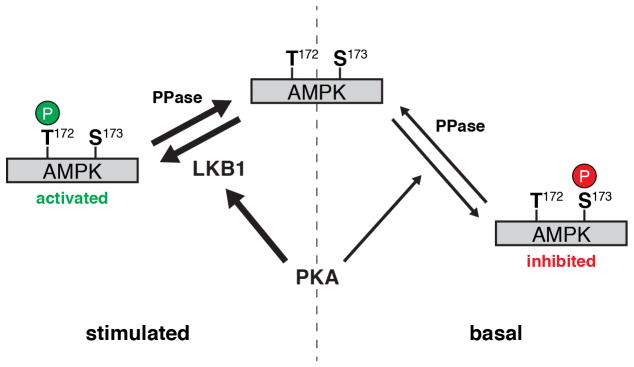
Figure 6.
Model of AMPK regulation by PKA. In resting (i.e., fed) cells, AMPK undergoes rapid basal phosphorylation at Thr172 by activating kinases and weak basal phosphorylation by PKA at inhibitory sites (here represented by Ser173). Whereas Thr172 is rapidly dephosphorylated by cellular phosphatase activity (PPase), inhibitory phosphorylation by PKA is only weakly antagonized, leading to the accumulation of a stable pool of inactivated AMPK. At the same time, however, starvation or PKA stimulation preferentially induces the phosphorylation of LKB1, leading to the robust activation of naïve AMPK via Thr172 phosphorylation.