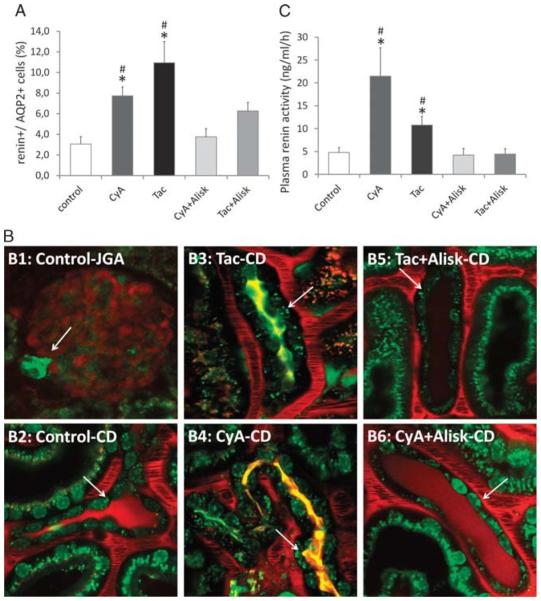
FIGURE 1.
Increased renin content in the collecting duct and its systemic effect. A, Renin content in principal (AQP2+) cells: approximately 3% of AQP2+ principal cells were positive for renin in control mice. After 3 weeks of CNI treatment, we have seen a significant increase in renin content, which diminished when the CNI treatment was combined with the direct renin inhibitor Aliskiren. B, Renin content in the collecting ducts: the classical renin-producing granular cells in the juxtaglomerular apparatus (B1: control-JGA) stained with Quinacrine in green (arrows). In the collecting duct, the control group (B2: control-CD) shows renin granules in trace (arrows), whereas the 2 CNI-treated groups presented robust expression of renin (B3: Tac-CD, B4: CyA-CD), which was almost completely abolished by the direct renin inhibitor Aliskiren (B5: Tac + Alisk-CD, B6: CyA + Alisk-CD). C, Serum renin activity: the control mice showed serum renin activity 4.8 ng/mL per hour. After 3 weeks of CNI treatment, we have seen a significant increase in renin activity, which diminished when the CNI treatment was combined with the direct renin inhibitor Aliskiren. (n = 4-6/group) *P ≤ 0.05 versus control, #P ≤ 0.05 versus Alisk; green: quinacrine (renin-positive granules), Hoechst 33342 (nuclei), and autofluorescence; red: 70 kDa rhodamine dextran (vasculature).