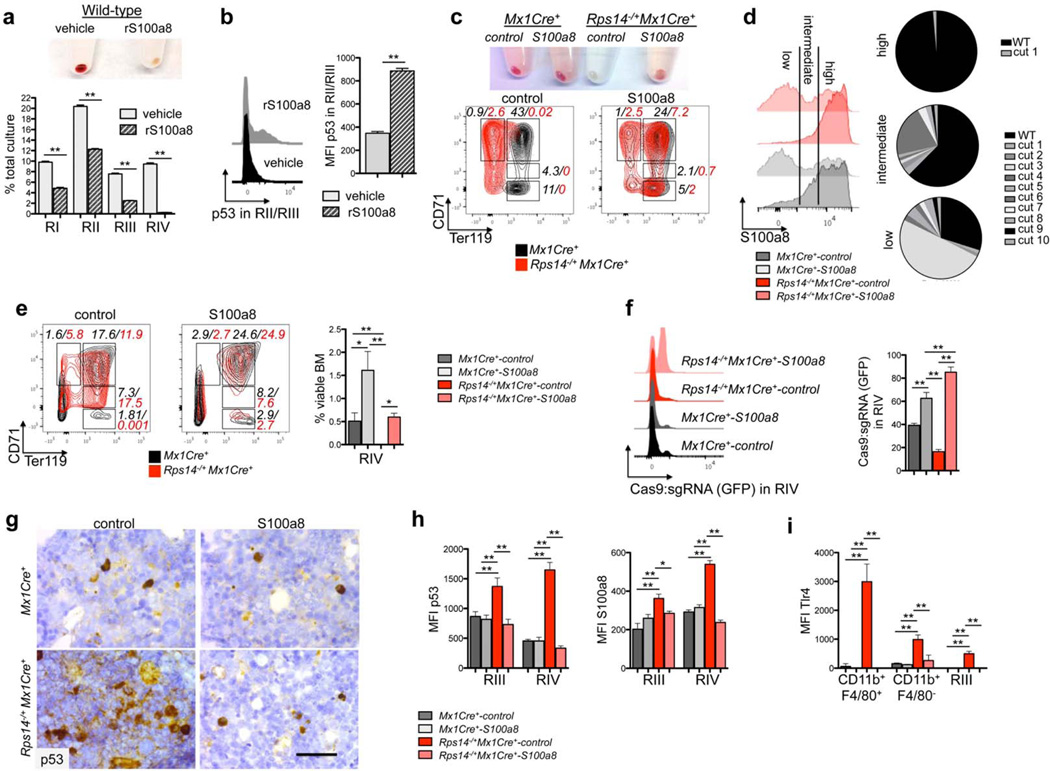
Figure 5. S100a8 is essential for the erythroid differentiation defect due to Rps14 haploinsufficiency.
(a) Lineage negative wild-type HSPCs were subjected to erythroid differentiation for 5 days in the presence of vehicle or S100a8 recombinant protein (rS100a8). Cell pellets 5 days after induction of differentiation (representative picture of 5 biological replicates is shown) and frequency of RI-RIV erythroid progenitor populations in the culture. (mean±SD, n=5 biological replicates; **p<0.001). (b) Intracellular flow cytometry for p53 expression within the RII/RIII populations in erythroid differentiation culture in vitro in presence or absence of recombinant S100a8. Representative histograms of p53 expression and mean fluorescence intensity of p53 within this population (mean±SD, n=5 biological replicates; **p<0.001). (c) Cell pellets of ckit+ HSPCs from Rps14−/+Mx1Cre+ or Mx1Cre+ mice expressing S100a8 or control sgRNAs following erythroid differentiation in vitro. Representative flow plots of the erythroid progenitor populations characterized by CD71 and Ter119 expression (RI-RIV); (data representative from n=3 biological replicates are shown). (d) Representative histogram presentation of S100a8 expression in Gr1lowCD11b+ monocytes in the erythroid differentiation culture in vitro after 5 days from Rps14−/+Mx1Cre+ or Mx1Cre+ cells which were transduced with either S100a8 or control sgRNAs. The pie chart represents the mutations introduced by the S100a8 sgRNA:Cas9 transduced cells after sequencing of S100a8 high, intermediate, and low fractions. (e) ckit+ HSPCs were transduced with a lentiviral vector expressing Cas9 and an sgRNA targeting S100a8 or control sgRNAs (non-targeting guide, NTG) and were transplanted 24 hours after infection into lethally irradiated wild-type recipients. 6 weeks after transplantation, recovered hematopoiesis in the transplanted mice was confirmed and PH was injected to induce hemolysis. 6 days after the first dose of PH, the bone marrow was harvested. Representative flow plots of the RI - RIV erythroid progenitor populations (mean±SD, n=5; **p<0.001; *p<0.05). (f) Representative histograms depicting GFP expression, representing cells transduced with control- or S100a8 sgRNA:Cas9 in the RIV population (mean±SD, n=5; **p<0.001). (g) p53 immunohistochemistry in bone marrows from Rps14−/+Mx1Cre+ or Mx1Cre+ mice transduced with either control control or S100a8 sgRNA:Cas9. Scale bar: 50µm. (h) Quantification of the mean fluorescence intensity (MFI) of intracellular staining with p53 and S100a8 in erythroid progenitor populations (RIII and IV) in bone marrows from Rps14−/+Mx1Cre+ or Mx1Cre+ mice transduced with either control or S100a8 sgRNA:Cas9. (mean±SD, n=5; *p<0.05; **p<0.001). (i) Quantification of the mean fluorescence intensity (MFI) of intracellular staining with Tlr4 in macrophages (CD11b+F4/80+), monocytes (CD11b+F4/80−) and in erythroid progenitor populations (RIII) in bone marrows from Rps14−/+Mx1Cre+ or Mx1Cre+ mice transduced with either control or S100a8 sgRNA:Cas9. (mean±SD, n=5; *p<0.05; **p<0.001). Unpaired two-sided t-test (a–b) or multiple group comparison (e–i) using analysis of variance with posthoc Tukey correction were applied for statistical analysis.