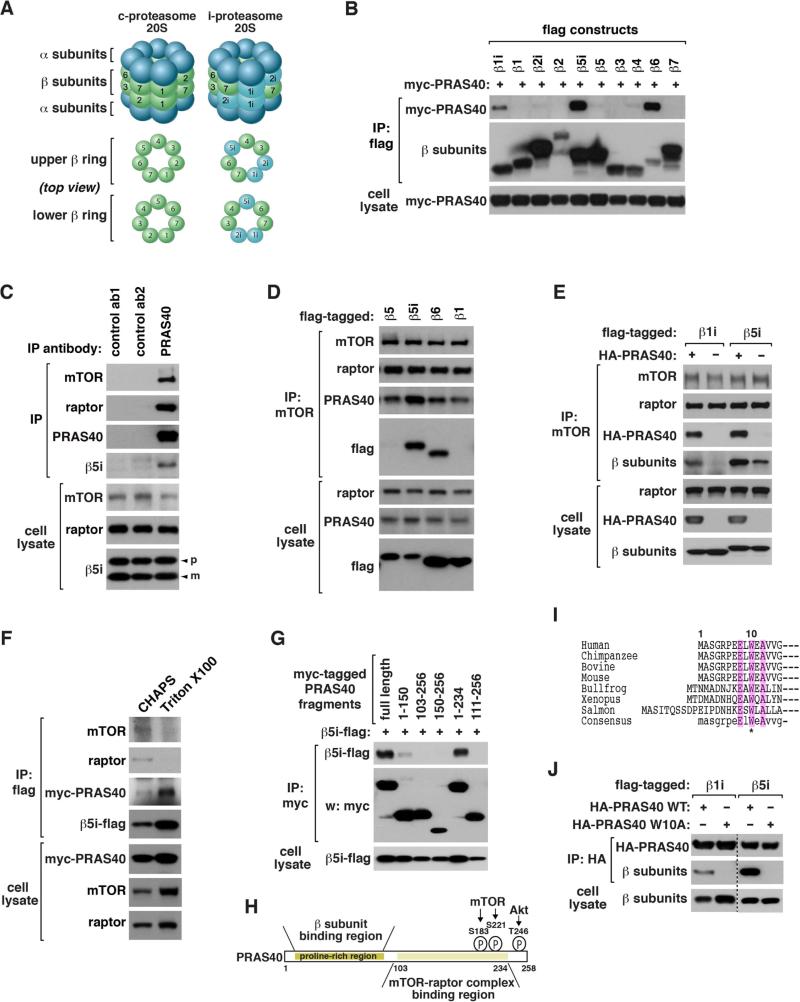
Figure 1. mTORC1 binds to i-proteasomal β1i, β5i and β6.
(A) Comparison of c-proteasome and i-proteasome 20S.
(B) β1i, β5i and β6 bind to PRAS40. C-terminal flag-tagged β subunits were expressed with myc-PRAS40 in HEK293T cells. Myc-PRAS40 recovered with flag immunoprecipitates was analyzed by western blotting (WB).
(C) Endogenous PRAS40 interacts with β5i. An untagged form of β5i was expressed in HEK293T cells. IP was conducted using antibodies for PRAS40, insulin receptor β (ab1) or GAPDH (ab2). “p” and “m” indicate the precursor and mature forms, respectively.
(D) Endogenous mTOR interacts with β5i and β6. C-terminal flag-tagged β subunits were expressed in HEK293T cells.
(E) PRAS40 enhances binding of mTOR to β1i or β5i. Flag-tagged β1i or β5i was expressed with or without HA-PRAS40 in HEK293T cells.
(F) Triton X100 does not disrupt the PRAS40-β5i interaction. Flag-β5i and myc-PRAS40 were transiently expressed in HEK293T cells. IP was conducted in the presence of CHAPS or Triton X100.
(G) β5i binds to an N-terminal region of PRAS40. The indicated constructs were expressed in HEK293T cells.
(H) PRAS40 uses two separate regions for binding to iβ subunits and mTOR/raptor. mTOR and Akt target sites are located near the C-terminus.
(I) N-terminal sequences of PRAS40 from different species.
(J) W10A disrupts binding of PRAS40 to β1i and β5i. Co-IP was conducted using proteins expressed in HEK293T cells.
See also Figure S1.