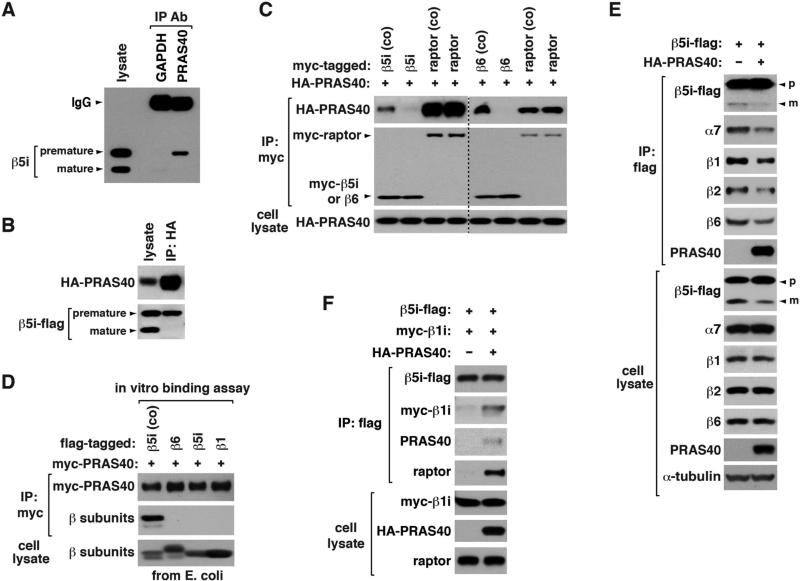
Figure 2. PRAS40 binds to iβ precursors during their folding and suppresses de novo assembly of the 20S core.
(A) Endogenous PRAS40 binds to β5i precursor. An untagged form of β5i was expressed in HEK293T cells, and its binding to PRAS40 was analyzed by co-IP. GAPDH IP was analyzed as control.
(B) β5i-flag was expressed with HA-PRAS40 in HEK293T cells.
(C) PRAS40 binds to β5i and β6 precursors during their folding. For coexpression (co), myc-tagged β subunit or raptor was coexpressed with HA-PRAS40 in HEK293T cells. Otherwise, myc-tagged construct was expressed in HEK293T cells then combined with cell lysate containing HA-PRAS40.
(D) PRAS40 can interact with β5i precursor without other components of mTORC1. For coexpression (co), β5i-flag was expressed with myc-PRAS40 in E. coli. Otherwise, flag-tagged β subunits were expressed in E. coli then combined with E. coli extracts containing myc-PRAS40.
(E) PRAS40 suppresses de novo biogenesis of proteasomes. β5i-flag was expressed with or without HA-PRAS40 in HEK293T cells. Proteasomal components associated with β5i-flag were analyzed by WB.
(F) PRAS40 enhances binding of β5i to β1i. Proteins were transiently expressed in HEK293T cells.
See also Figure S2.