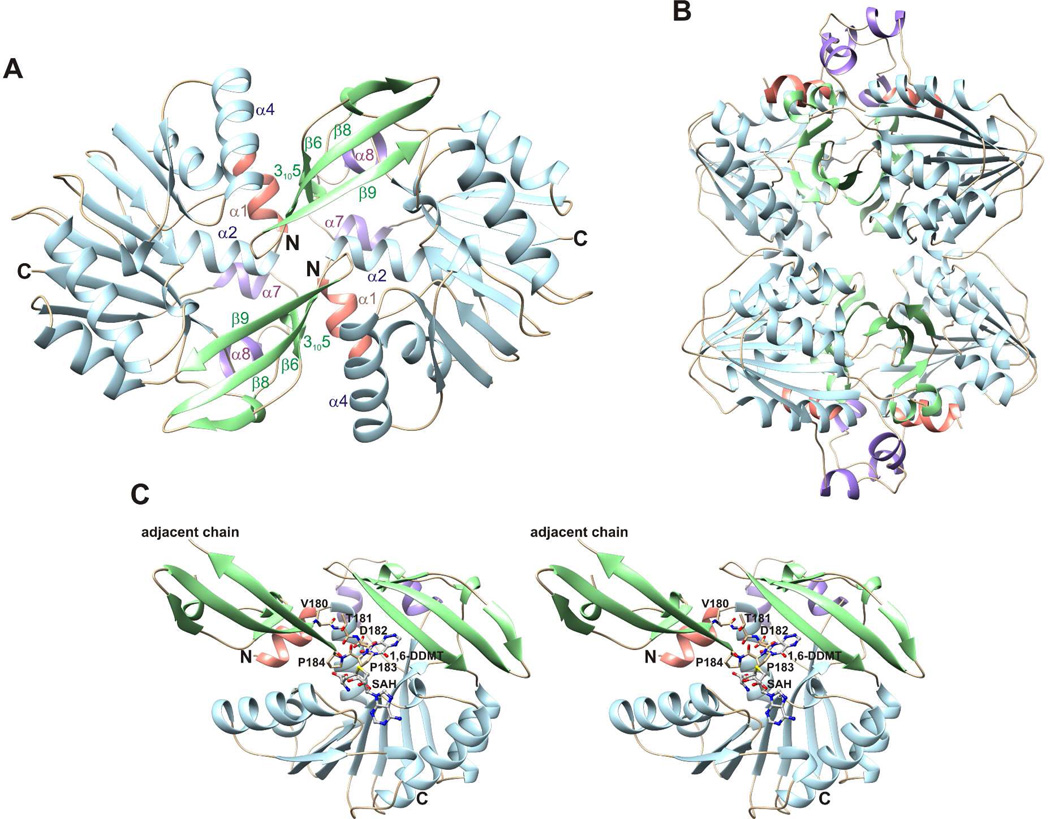
Figure 4.
Crystal structure of apo ToxA. (A) Apo ToxA forms a homodimer having noncrystallographic twofold symmetry. (B) Homotetramic arrangement of apo ToxA observed within the crystal packing. Two dimers having the structure shown in panel A form a homotetramer with 222 symmetry. (C) A flexible loop (Val180-Thr181-Asp182-Pro183-Pro184) from the adjacent chain inserts into the azapteridine binding site and prevents the N-terminal segment from folding over the active site. 1,6-DDMT and SAH are superimposed onto the apo structure to indicate potential steric clashes.