Fig. 2.
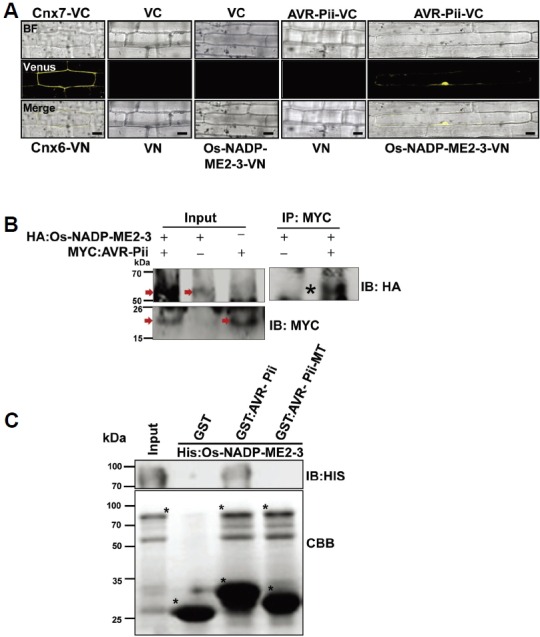
Validation of AVR-Pii and Os-NADP-ME2-3 interactions. (A) BiFC assay to validate the interaction of AVR-Pii (bait) and Os-NADP-ME2-3 (prey). Both bait and prey were fused to the C-and N-terminal halves of Venus and transformed to rice leaf sheaths using biolistic bombardment as described in the Materials and Methods section. The signal was detected using a Venus filter (Ex/Em: 488nm/505-550 nm wavelength) in a confocal microscope (Leica, TCS SP5). (B) AVR-Pii (without signal peptide) and Os-NADP-ME2-3 interaction was determined in planta by Co-IP. The protein extract was isolated from Nicotiana benthamiana leaves expressing the HA:Os-NADP-ME2-3 and Myc:AVR-Pii transgenes and protein expression was checked by anti-HA and anti-myc antibody respectively as shown by arrow in the Figure. The extract was immuno-precipitated by anti-Myc antibody and subjected to immunoblotting by anti-HA antibody. Asterisk indicates co-immuniprecipitated band of HA:Os-NADP-ME2-3 detected by anti-HA antibody. (C) In vitro GST pull-down assay of recombinant GST:AVR-Pii bound to rice 6xHis: Os-NADP-ME2-3. The pull-down and input samples were subjected to CBB staining as well as immunoblotting by the anti-His antibody (1:1000; ABM Inc., GO20). The result demonstrated that AVR-Pii genuinely interacts with Os-NADP-ME2-3, and mutated AVR-Pii (AVR-Pii-MT) fails to interact. The construction of AVR-Pii-MT is described in the Materials and Methods section. The asterisk (*) represents Histagged Os-NADP-ME2-3 (upper), GST alone, GST-tagged AVR-Pii and AVR-Pii-MT (lower). IP, Immunoprecipitation; IB, immunoblotting; CBB, Coomassie Brilliant Blue; VC, pDEST-VYCE®GW; VN, pDEST-VYNE®GW. Scale bars, 10 μm.
