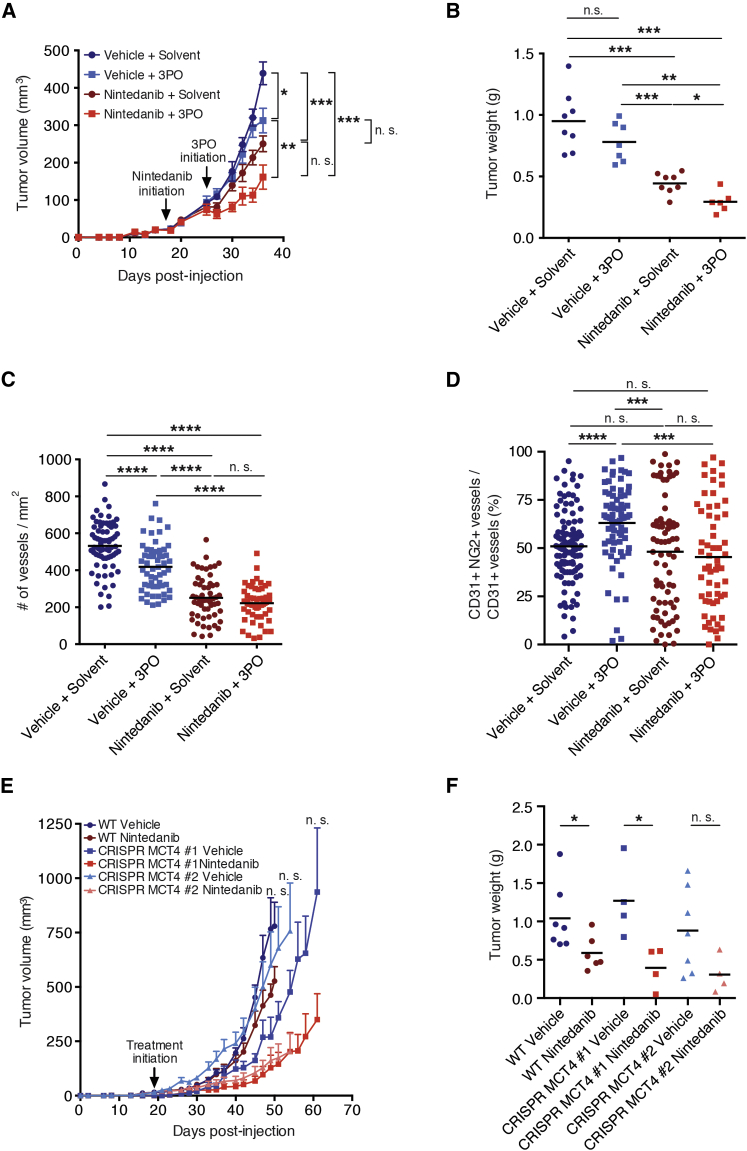
Figure 5.
Targeting Glycolysis or Metabolic Symbiosis in Combination with Nintedanib Treatment Delays Tumor Growth
(A and B) Primary tumor growth over time (A) and tumor weights at the experimental endpoint (B) of mice treated with either vehicle or nintedanib (50 mg/kg/day) in combination with 3PO (70 mg/kg/day) or solvent are shown. 3PO treatment was initiated 8 days after the initiation of nintedanib treatment and then continued as combinatorial treatment (LT treatment). In (A), data are displayed as mean tumor volumes ± SEM.
(C) Quantification of microvessel densities by immunofluorescence staining for CD31 on histological tumor sections from LT nintedanib and 3PO-treated mice. Values represent the number of counts per each area of microscopic field of view, and means are displayed. n = 6–8 mice per group.
(D) Pericyte coverage was assessed by immunofluorescence staining for CD31 and NG2 on histological tumor sections from LT nintedanib and 3PO-treated mice. Values represent the percentage of NG2+ blood vessels, and means are displayed. n = 4–5 mice per group.
(E and F) Primary tumor growth over time (E) and tumor weights at the experimental endpoint (F) of mice injected with Py2T wild-type (WT) or Py2T CRISPR MCT4 no. 1 and no. 2 cells and treated with either vehicle control or nintedanib (50 mg/kg/day) are shown. Nintedanib treatment was initiated 19 days after tumor cell injection, once the tumors were palpable. Mice injected with CRISPR MCT4 no. 1 cells presented a delayed tumor onset and were therefore treated once the tumors became palpable (days 27–38). In (E), data are displayed as mean tumor volumes ± SEM. n = 4–7 mice per group.
Mann-Whitney U test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. See also Figure S5.